Figures & data
Figure 2. Some benzoxazine structures of ADP and/or collagen-induced platelet aggregation, DNA-PK and PI3K inhibitors.
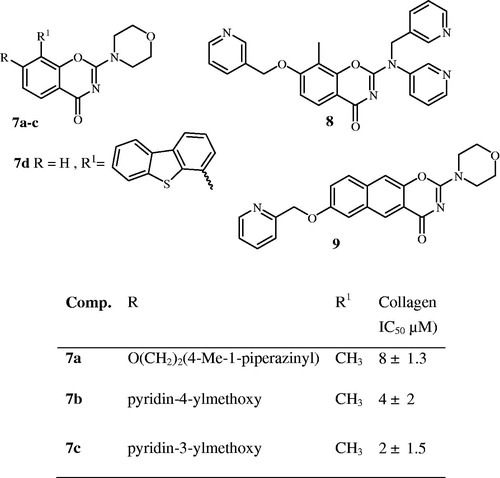
Table 1. PI3K inhibition IC50 of product 12b, 13b, 15a and 17b,c.
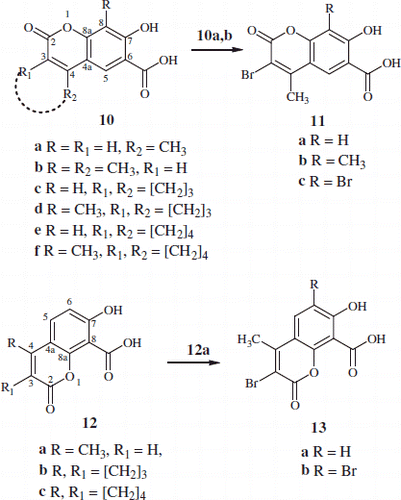
Scheme 1. Synthesis of substituted-7-hydroxy-2-oxo-2H-chromene-6-carboxylic acid 10a–f, 11a–c and chromene-8-carboxylic acids 12a–c & 13a,b.
Scheme 2. Synthesis of substituted-2-thioxo-chromen-1,3-oxazine linear compounds 14, angular compounds 16, 2-morpholino-substituted-chromen[6,7-e][1,3]oxazine-4,8-dione 15 and 8-morpholino-substituted-chromen[8,7-e][1,3]oxazine-2,10-dione 17. Reaction conditions: (i) freshly prepared Ph3P(SCN)2; (ii) morpholine in dioxane/reflux.
![Scheme 2. Synthesis of substituted-2-thioxo-chromen-1,3-oxazine linear compounds 14, angular compounds 16, 2-morpholino-substituted-chromen[6,7-e][1,3]oxazine-4,8-dione 15 and 8-morpholino-substituted-chromen[8,7-e][1,3]oxazine-2,10-dione 17. Reaction conditions: (i) freshly prepared Ph3P(SCN)2; (ii) morpholine in dioxane/reflux.](/cms/asset/eafdb003-f9f9-4040-9337-2d6e764369d3/ienz_a_1190710_sch0002.gif)
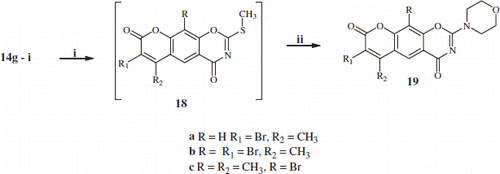
Scheme 3. Synthesis of mono- and di-bromo-2-morpholino-substituted-benzoxazines 19a–c. Reaction condition: (i) NaHCO3\in water/2-propanol/MeI; (ii) morpholine/RT.
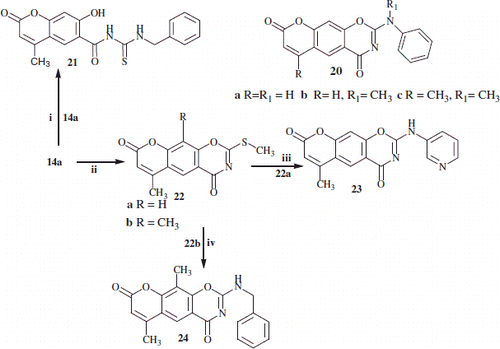
Scheme 4. Outline the reactions of substituted-2-thioxo and 2-methyl thio-chromen-1,3-oxazine linear compounds 14a,b and 22a,b with benzylamine and 2-(pyrdin-3-yl-amino) respectively. Reaction conditions: (i) NaHCO3/benzylamine in water/2-propanol; (ii) NaHCO3 in water/2-propanol/MeI; (iii) 3-aminopyridine; (iv) benzylamine.