Figures & data
Table 1. Method I and II for the synthesis of dipolarophiles 2–6a.
Table 2. Optimization of the 1,3-dipolar cyclization for the ruthenium-catalyzed click reactionTable Footnotea.
Scheme 1. Synthesis of the 1,5- and 1,4-triazolyl (8a–g, 9a–g) derivatives. Reagents and conditions: (a) [Cp*RuCl(PPh3)2], DMF, Microwave (250 W, 4–8 min), 83–98%. (b) Cuprous iodide (CuI), Et3N, solvent free, Microwave (200 W, 2–4 min), 90–99%.
![Scheme 1. Synthesis of the 1,5- and 1,4-triazolyl (8a–g, 9a–g) derivatives. Reagents and conditions: (a) [Cp*RuCl(PPh3)2], DMF, Microwave (250 W, 4–8 min), 83–98%. (b) Cuprous iodide (CuI), Et3N, solvent free, Microwave (200 W, 2–4 min), 90–99%.](/cms/asset/7caf71f1-f980-470b-a715-1baeda3eb7aa/ienz_a_1193733_sch0001.gif)
Table 3. Maslinic acid-1,5- and 1,4-disubstituted triazolyl derivatives varying at aromatic ring.
Table 4. Optimization of CuAAC under microwave conditions for 9aa.
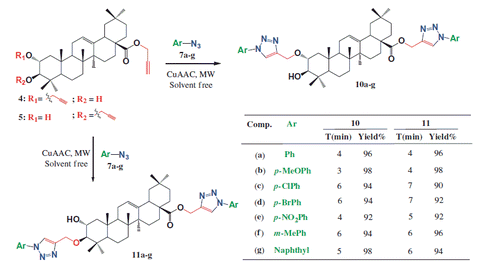
Scheme 2. Bis-1,4-disubstituted triazoles 10a–g and 11a–g prepared by the Cu(I)-catalyzed synthesis.