Figures & data
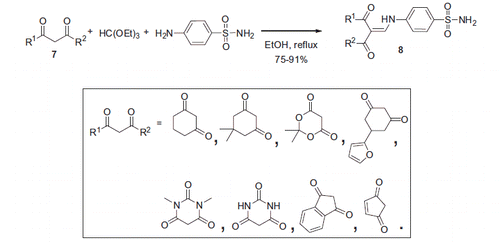
Figure 1. Most potent CA inhibitors reported by Allouche et alCitation16.
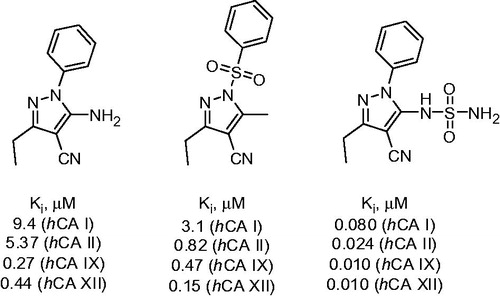
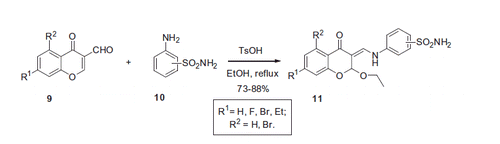
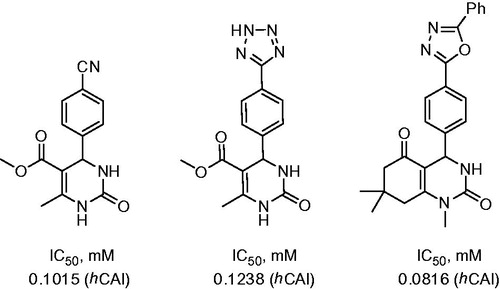
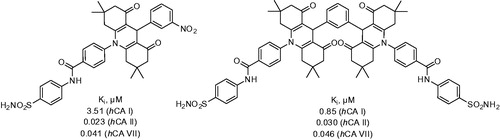
Figure 2. Most potent inhibitors of hCA I and hCA II, respectively, reported by Dimirci et alCitation19.
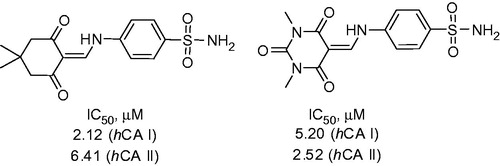
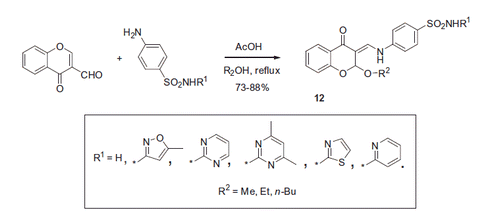
Figure 3. Biological activity data for the lead compound 12 reported by Awadallah et alCitation21.
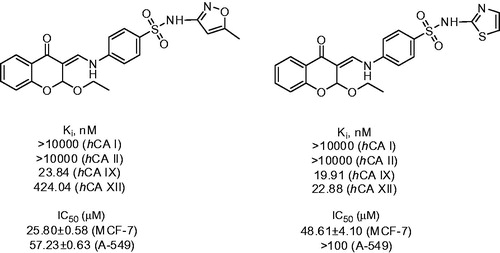
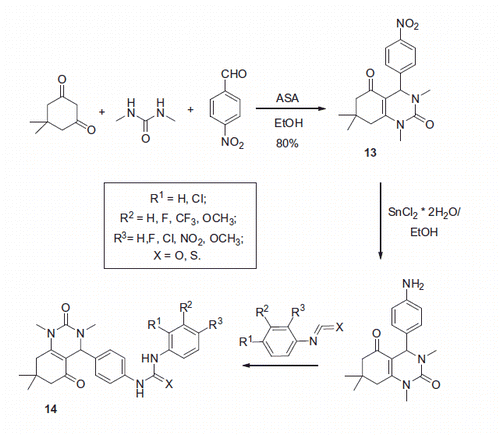
Figure 4. Inhibitory properties of exemplary urea and thiourea derivatives reported by Celic et alCitation22.
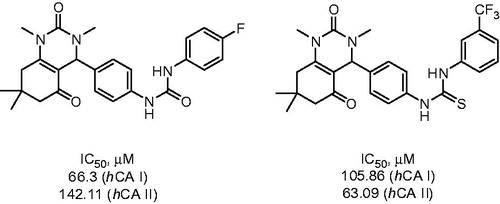
Figure 6. Most active compounds of 28 phtalazine derivatives reported by Beber et alCitation24,Citation25.
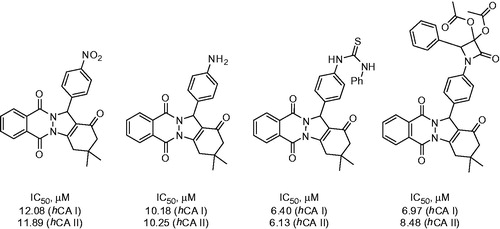
Figure 7. Leading compound reported by Suthar et alCitation28.
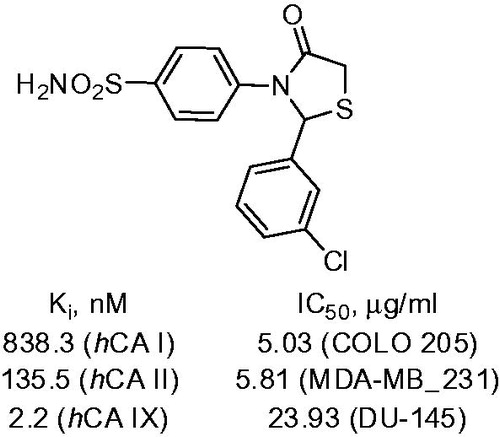
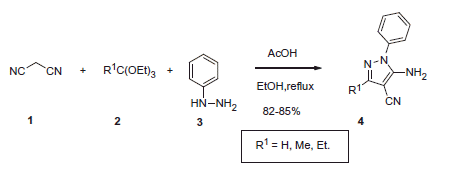
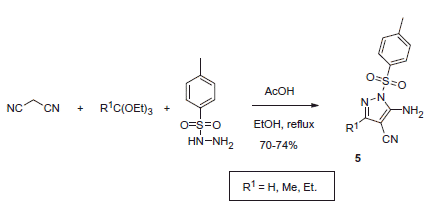
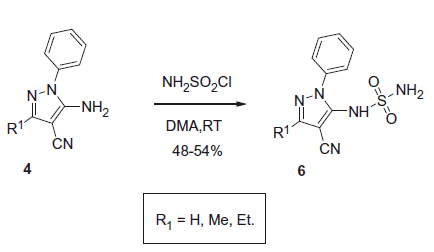
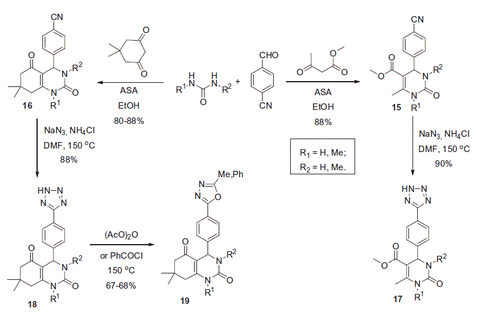
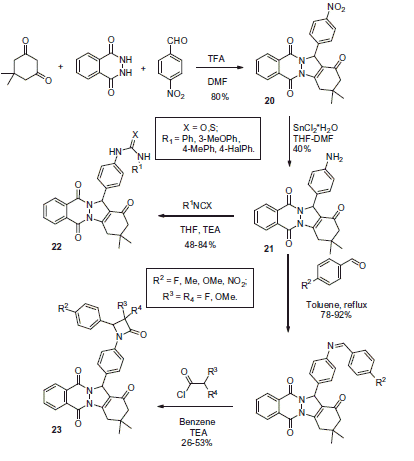
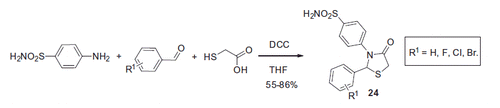
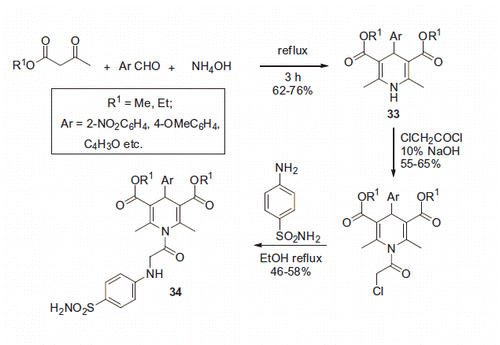
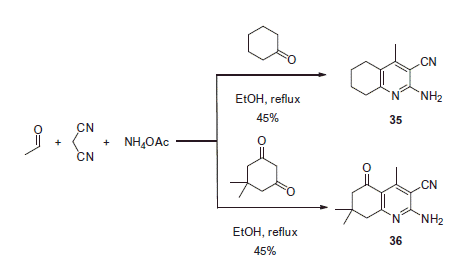
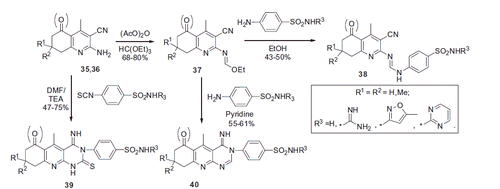
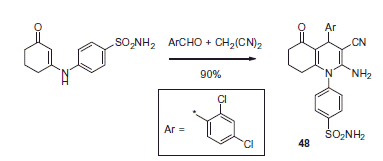
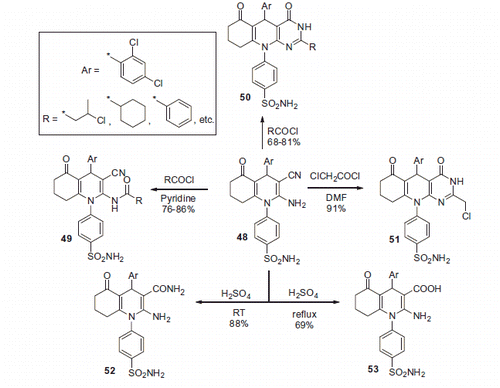
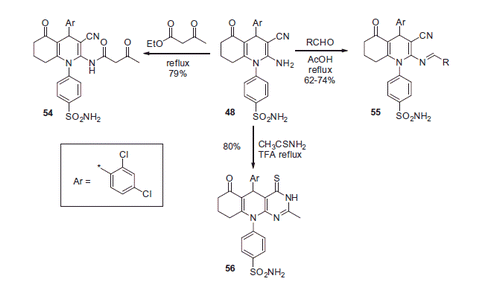
Scheme 17. Examples of derivatization of 2-amino-3-cyano-1,4-dihydropyridine moiety in sulfonamide 41.
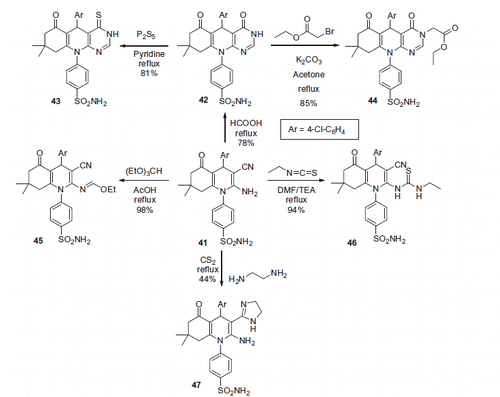
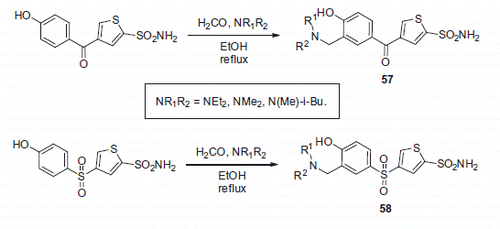
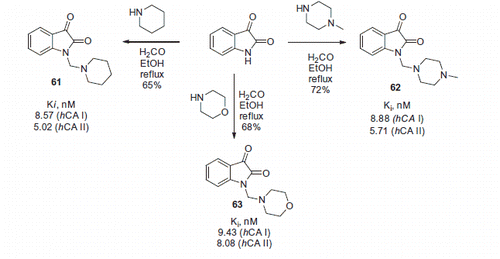
Figure 10. Most active Mannich adducts, reported by Hartman et alCitation39.
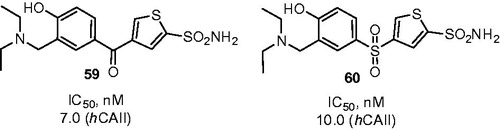
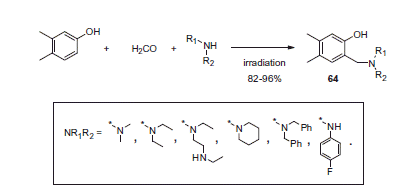
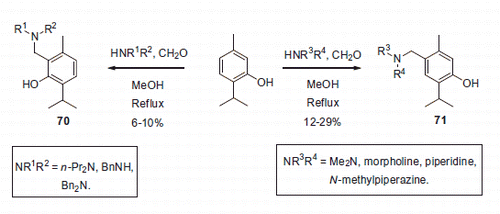
Figure 11. Mannich adducts reported by Chow et alCitation42.
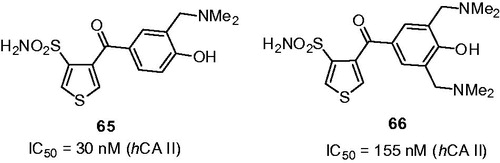
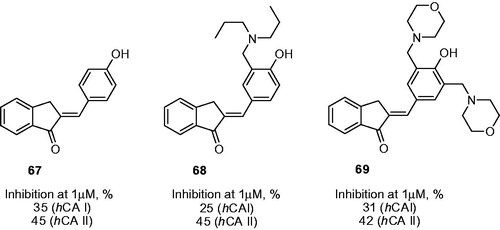
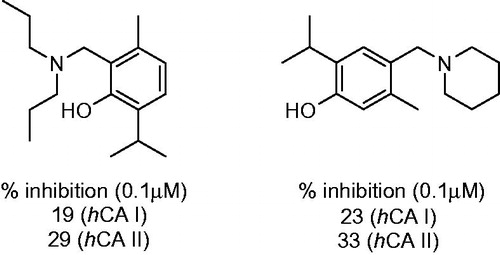
Figure 12. Percentage of inhibition data for progenitor compound 67 and its most potent Mannich derivatives 68–69.