Figures & data
Table 1. (a) Structures and experimental inhibitory potency (Ki, nM) for compounds of the general structures Ι and II.
Table 1. (b) Structures and experimental inhibitory potency (Ki, nM) for compounds of the general structures III, IV, and V.
Table 1. (c) Structures and experimental inhibitory potency (Ki, nM) for compounds of the general structures VI, VII, and VIII.
Table 2. Summary of the results obtained from the CoMFA, CoMFA-RF, and CoMSIA models.
Table 3. Experimental and predicted inhibitory activities (pKi) with residual values for the training and test set compounds.
Figure 1. CoMFA contour maps based on compound 26: (a) steric, (b) electrostatic, and (c) consolidated steric and electrostatic.
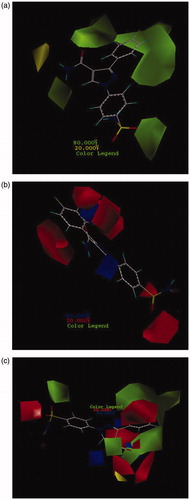
Table 4. Structural form of the more active compounds than acetazolamide (AZA).
Figure 2. CoMSIA contour maps for hydrophobic and hydrophilic features: (a) for compound 26 and (b) for more active compounds than AZA.
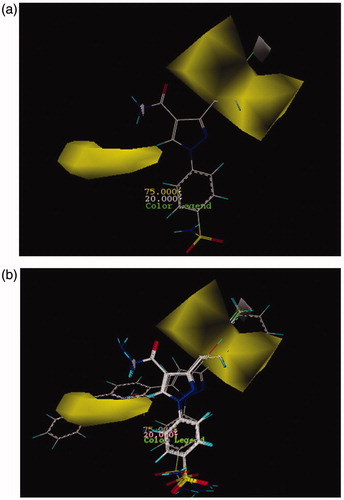
Figure 3. CoMSIA contour maps for the absence and the presence of hydrogen bond donor features based on (a) compound 26 and (b) more active compound than AZA.
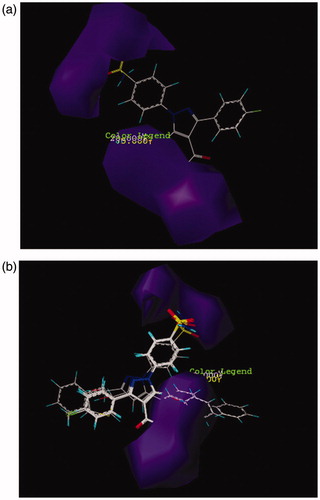
Figure 4. CoMSIA contour maps for the absence and the presence of hydrogen bond acceptor features based on (a) compound 26 and (b) more active compound than AZA.
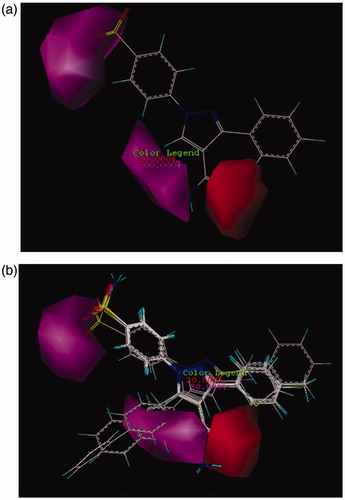
Table 5. Structures and GOLD fitness score values for the hit compounds.
Table 6. Prediction of ADME properties of hits using Qikprop.
Table 7. Prediction of drug likeness parameters and assessment of risk factors by OSIRIS Property Explorer.
