Figures & data
Figure 1. Best docking pose found between chlorogenic acid and tyrosinase (A), α-glucosidase (B), butyrylcholinesterase (C) and a-amylase (D).
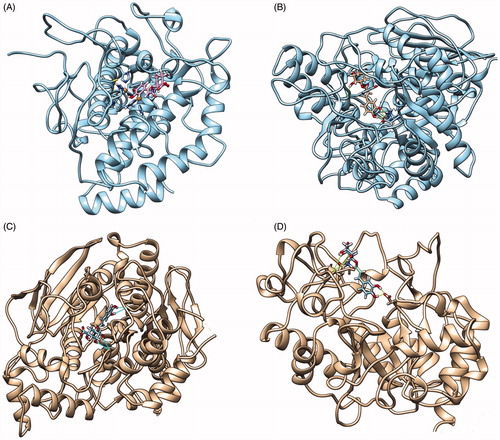
Figure 2. Total phenolic and flavonoid contents of the three extracts from Lycium barbarum leaves (mean ± SD).
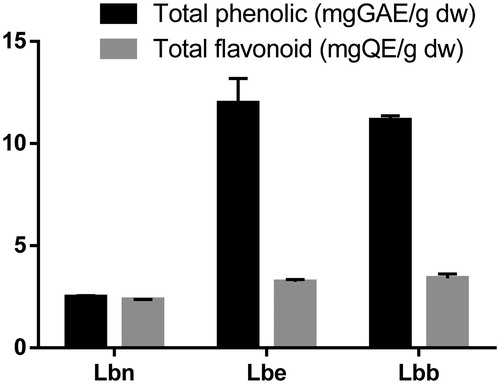
Figure 3. LC-DAD chromatogram (at 325 nm) of the phenolic compounds from L. barbarum leaves (cultivar Lbb- "Bigligeberry"). For compounds numbers refer to .
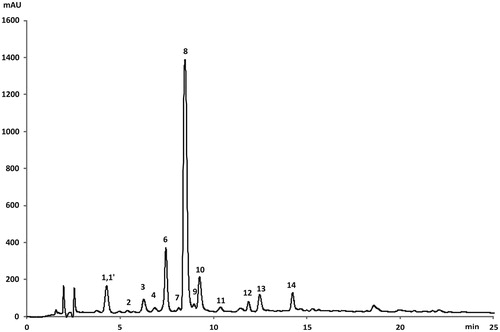
Table 1. Phenolic compounds in Lycium barbarum leaves, retention times (Rt), wavelengths of maximum absorption (λmax), mass spectral data and identification.
Table 2. Phenolic compounds (μg/g dw) and their distribution in Lycium barbarum leaves (mean ± SD).
Figure 4. Total antioxidant capacity of Lycium barbarum leaves measured with TEAC and EPR spectroscopy. Results were expressed as mg TE/g dw, and as mg FSE/g dw. The error bars are the result of a triple determination.
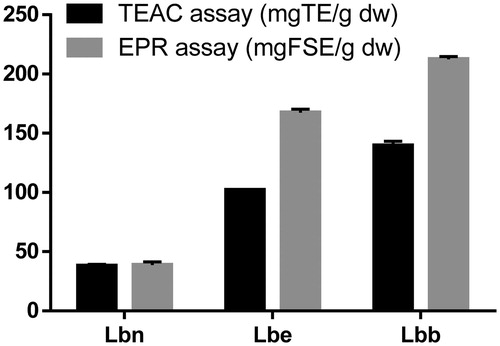
Table 3. The enzyme inhibitory effects of Lycium leaves.
Figure 6. Binding interactions of the best pose of chlorogenic acid in complex with: α-glucosidase (A), butyrylcholinesterase (B), and α-amylase (C) (cut-off 4 Å).
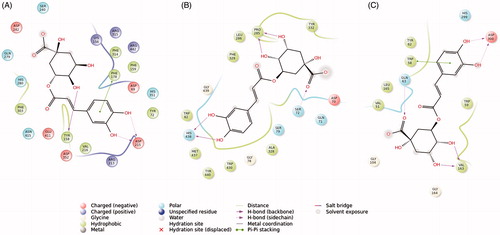
Figure 7. RMSD (in Angstrom) time-dependent plot of chlorogenic acid fluctuation docked to tyrosinase (A); close-up of the complex chlorogenic acid-tyrosinase (hydrogen bonds are reported as green lines) (B); details of the interactions formed by chlorogenic acid with the residues present in the binding pocket (cut-off = 4 Å from the ligand) (C).
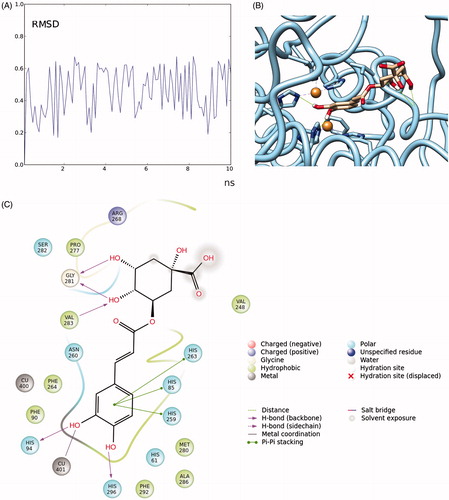
Table 4. Antimicrobial activity of the three Lycium leaves extracts.
Table 5. Antimutagenic properties of Lycium leaves extracts on Salmonella typhimurium TA 98 and TA 100.