Figures & data
Figure 1. Structures of azopirones (buspirone, gepirone, ipsapirone, tandopirone and zalospirone) and general structrure of the synthesized compounds (2a-n).
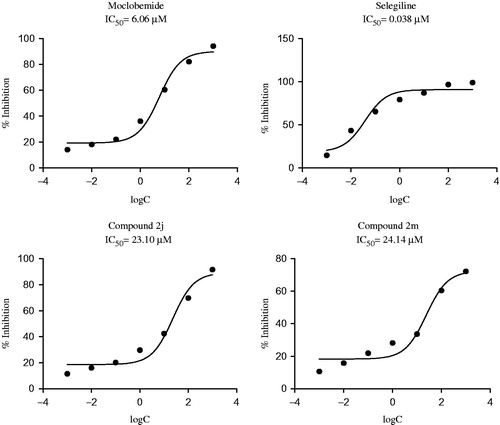
Figure 2. Lineweaver–Burk plots for compound 2j (IC50 = 23.10 μM). Substrate (kynuramine) concentrations used: 40, 20, 10, 5, 2.5 and 1.25 μM. 1/V: 1/velocity of reaction [1/(nmoles/min/mg protein)], 1/S: 1/substrate concentration (1/μM).
![Figure 2. Lineweaver–Burk plots for compound 2j (IC50 = 23.10 μM). Substrate (kynuramine) concentrations used: 40, 20, 10, 5, 2.5 and 1.25 μM. 1/V: 1/velocity of reaction [1/(nmoles/min/mg protein)], 1/S: 1/substrate concentration (1/μM).](/cms/asset/dd219ca7-231a-4e96-9207-e24d2bba4692/ienz_a_1247054_f0002_b.jpg)
Table 1. In silico physicochemical parameters of the compounds 2a-n.
Figure 3. Synthesis of the compounds 2a-n. Reactants, reagents and conditions: i: ClCOCH2Cl, Et3N, THF, 0–5 °C, 3 h; ii: Potassium/Sodium salts of substituted piperazine dithiocarbamates, K2CO3, acetone, r.t, 5 h.
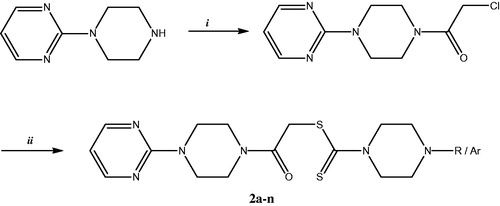
Table 2. Inhibitory activity (%) of the compounds against MAO-A enzyme.
Table 3. Inhibitory activity (%) of the compounds against MAO-B enzyme.