Figures & data
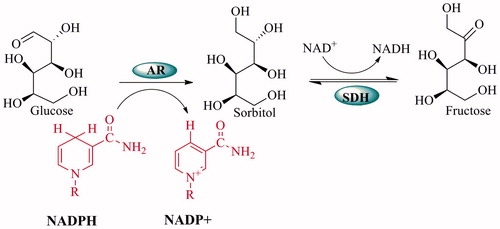
Figure 2. The relationship between oxidative stress and diabetic complications of the polyol pathway: Accelerated flux polyol pathway plays a critical role in the development of diabetic complications. Cataract is one of the diabetic complications. It is known that sorbitol accumulates in tissues and causes an increase in the osmotic pressure. Thus, water enters into the cells and swelling takes place, which can cause a cataract. Also, AR competes with glutathione reductase (GR) for their co-factor NADPH. The activity of GR is decreased when the activity of AR increased and this leading to a decrease in GSH level. Increased NADH causes NADH oxidase (NOx) to produce ROS. Fructose-3-phosphate (F-3-P) and 3-deoxyglucosone (3-DG), metabolites of fructose, increase AGE and binding of advanced glycation end product (AGE) to receptor of AGE (RAGE) increase oxidative stressCitation4.
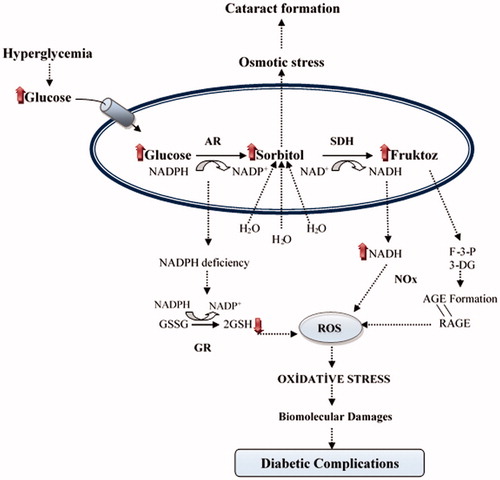
Figure 4. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis analysis of purified rat kidney aldose reductase. Lane 1: Standard proteins (kDa). Lane 2 and lane 3: Purified rat kidney aldose reductase enzyme.
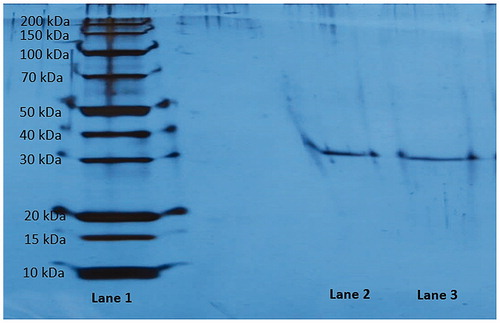
Table 1. Summary of purification of aldose reductase from rat kidney.
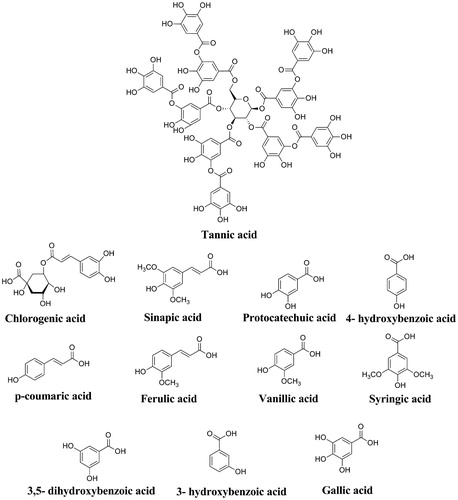
Table 2. IC50, Ki values and inhibition types for phenolic acids used in this study.
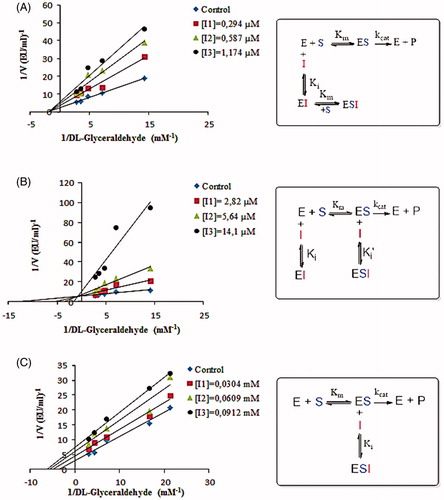
Figure 5. A: Lineweaver–Burk graph and inhibition mechanism of tannic acid using three different tannic acid concentrations for determination of Ki and inhibition type. B: Lineweaver–Burk graph and inhibition mechanism of chlorogenic acid using three different chlorogenic acid concentrations for determination of Ki and inhibition type. C: Lineweaver–Burk graph and inhibition mechanism of p-coumaric acid using three different p-coumaric acid concentrations for determination of Ki and inhibition type.