Figures & data
Table 1. IC50 values of anti-α-Gls activity of the compounds 1–13.
Figure 3. Cell survival rate diagram of compounds show survival of HeLa, A549 and MDA-MB-453 cells grown for 72 h in the presence of increasing concentrations of investigated compounds.
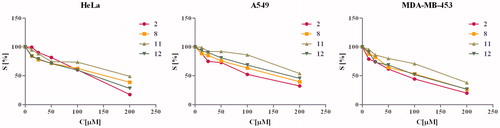
Table 2. IC50 values (μM) of tested compounds against malignant cell lines.
Figure 4. Morphological changes observed on HeLa cells after 24 hours treatment with IC50 and 2 × IC50 concentrations of compounds dilutions. (A) Control; (B) IC50, (C) 2 × IC50 (supernatant) compound 2; (D) IC50, (E) 2 × IC50 (supernatant) compound 8; (F) IC50, (G) 2 × IC50 (supernatant) compound 11; (I) IC50, (H) 2 × IC50 (supernatant) compound 12; fluorescent microscope (PALM MicroBeamsystems, Carl Zeiss, 20×)
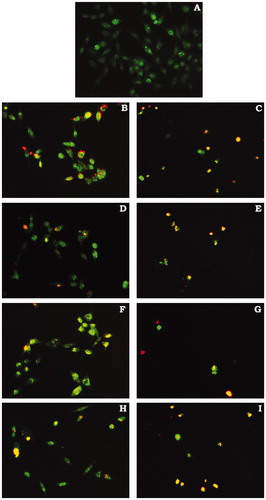
Table 3. Statistical performance of the final PLS model.