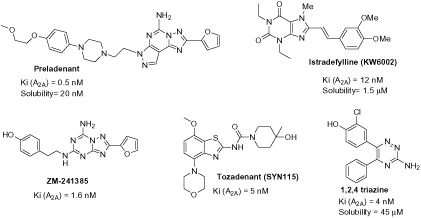
Figures & data
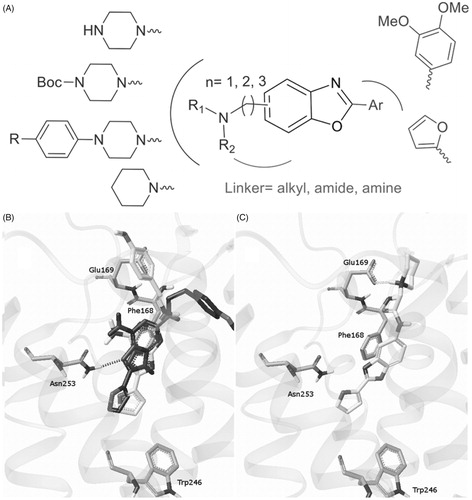
Figure 2. Molecular modelling-guided design. (A) Representation of various modulations around the benzoxazole scaffold. (B) Predicted binding mode of ZM-241385 in the apoA2AR-T4E pocket (dark) compared with the X-ray binding mode (gray). (C) Putative binding mode of compound F1 in the apoA2AR-T4E pocket.
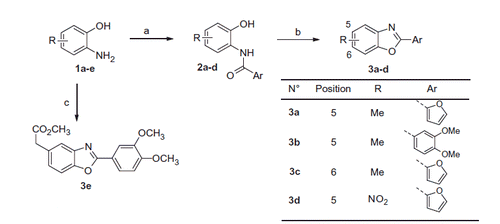
Scheme 1. Reagents and conditions: (a) i) ArCO2H, SOCl2, DMF, DCM, ii) Et3N, EtOAc, aminophenol (1a–d); iii) NaOH, EtOH/H2O, then 6 M HCl, 60–80% over 2 steps; (b) APTS, toluene, reflux, 70–80%, (c) T3P® (50% in EtOAc), DIPEA, 3,4-dimethoxybenzoic acid, 35%.
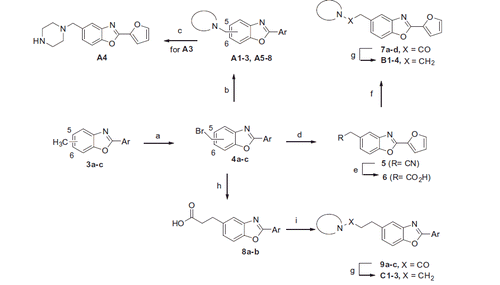
Scheme 2. Reagents and conditions: (a) NBS, benzoyl peroxide, CCl4, reflux/hυ (230 W), 60–75%; (b) R2R1NH, Et3N, acetone, 50–85%; (c) TFA, DCM, 60%; (d) KCN, EtOH/H2O, 50–60%; (e) H2O/H2SO4/AcOH, reflux, 60–70%; (f) i) SOCl2, toluene; ii) secondary amine, Et3N, EtOAc, 50–60%; (g) LiAlH4, THF, 60–75%; (h) i) dimethyl malonate, K2CO3, acetone; ii) NaOH, H2O then 6 M HCl; iii) DMF, reflux, 40–43%.
Scheme 3. Reagents and conditions: (a) LiAlH4, THF, 86%; (b) MsCl, Et3N, DCM, quant.; (c) secondary amine, K2CO3, DMF, 60 °C, 25–28%.
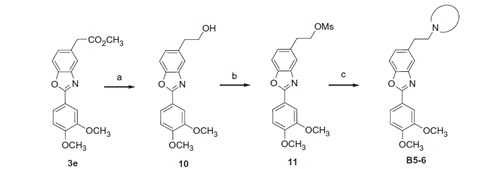
Table 1. A2A receptor affinity and cytotoxicity data of compounds A1–8, B1–6 and C1–3.
Scheme 4. Reagents and conditions: (a) hydrazine hydrate, Pd/C (10%), EtOH, 81%; (b) 1-(benzyloxy)-4-(2-bromoethyl)benzene, K2CO3, DMF, 70 °C, 15%; (c) H2, Pd/C (10%), MeOH, 48%; (d) N-chloroethylpiperidine, K2CO3, DMF, 70 °C, 8%; (e) bromoacetylbromide, NEt3, DCM, 75%; (f) for F1–3, secondary amine, K2CO3, acetone, 48–90%; (g) for F4, i) boc-piperazine, K2CO3, acetone, ii) 6 M HCl, MeOH, 72%.
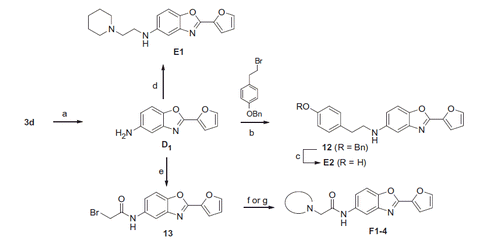
Table 2. A2A receptor affinity and cytotoxicity data of compounds D1, E1–2 and F1–4.
Table 3. Preliminary ADME studies of F1.