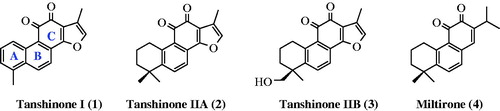
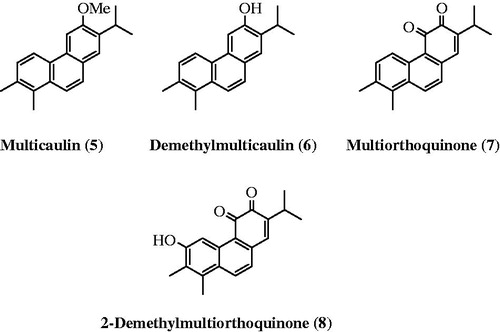
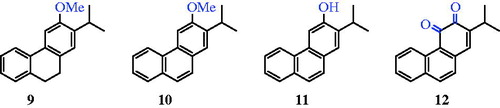
Figures & data
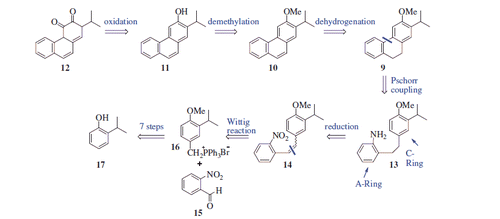
Scheme 2. Reaction conditions and reagents: (i) Me2SO4, NaOH (aq), 90 °C, 4 h, 93%; (ii) LiBr/(NH4)2Ce(NO3)6, CH3CN, 20 °C, 2 h, 97%; (iii) CuCN, DMF, 140 °C, 12 h, 90%; (iv) EtOH, H2SO4, reflux, 12 h, 84%; (v) LiAlH4, THF, 0 °C, 4 h, 91%; (vi) PBr3, DCM, 0 °C, 12 h, 92%; (vii) PPh3, CH3CN, reflux, 24 h; (viii) NaH, DCM, 0 °C, 15 min; and 2-nitrobenzaldehyde (15), 20 °C, rt, 16 h; and (ix) H2, Pd/C, MeOH, 20 °C, 2 h.
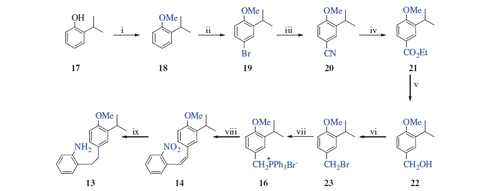
Scheme 3. Synthesis of 11 and 12a. aReaction conditions and reagents: (i) isopentyl nitrite, H2SO4, acetone, 0–10 °C, 2 h, 24%; (ii) DDQ, toluene, reflux, 24 h, 70%; (iii) BBr3, DCM, N2 atm., 0 °C, 12 h, 93%; (iv) DMP, DCM, 0 °C, 12 h, 61%.
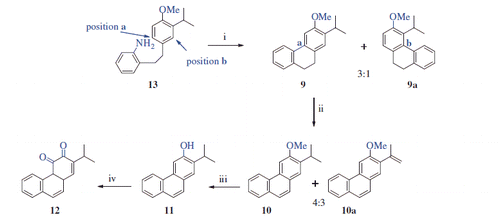
Table 1. In vitro antimycobacterial activity of synthesised compounds (9–12) expressed as the minimum inhibitory concentration (μm).