Figures & data
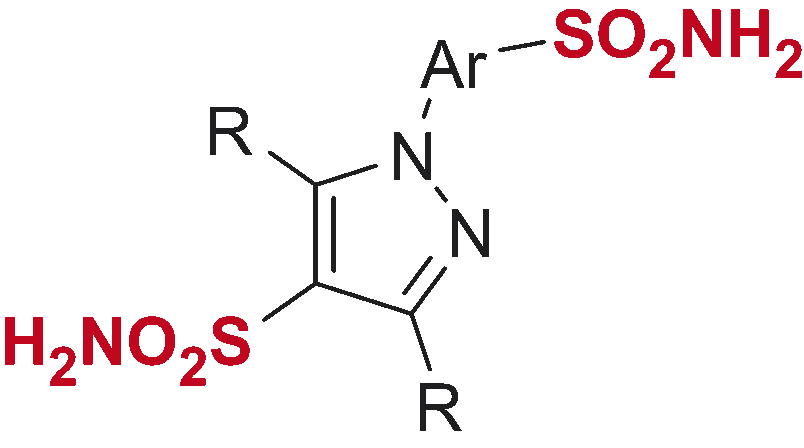
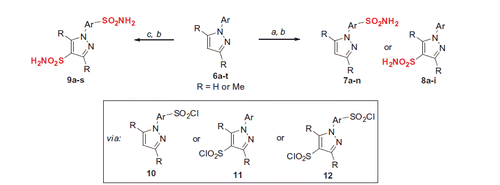
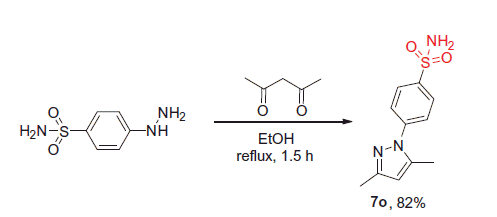
Figure 4. N-Arylpyrazole substrates 6a-t investigated in direct mono- and bis-sulfochlorination reactions (regiochemistry established for the respective mono- and bis-sulfonamides).
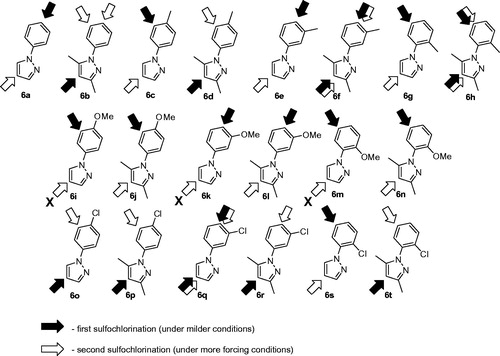
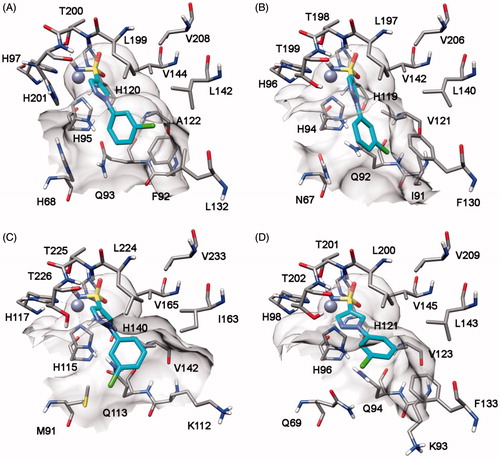
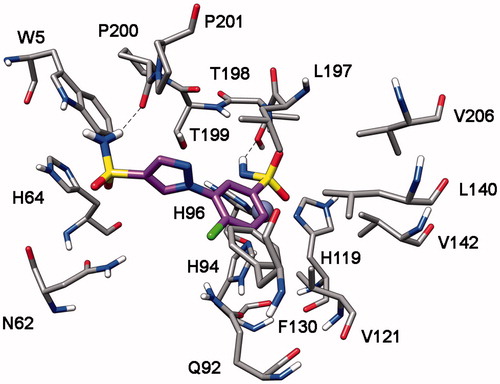
Table 1. Inhibitory profile of mono-sulfonamides 7a–o against four hCA isoforms.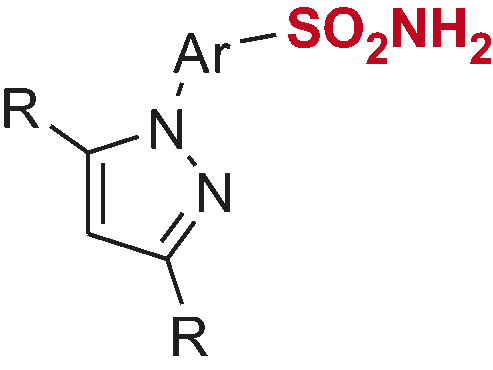
Table 2. Inhibitory profile of mono-sulfonamides 8a–i against four hCA isoforms.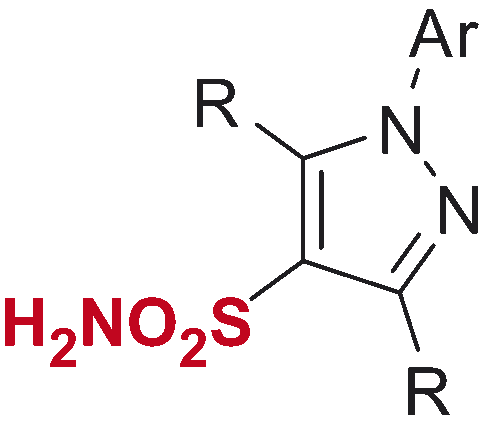
Table 3. Inhibitory profile of mono-sulfonamides 9a–s against four hCA isoforms.