Figures & data
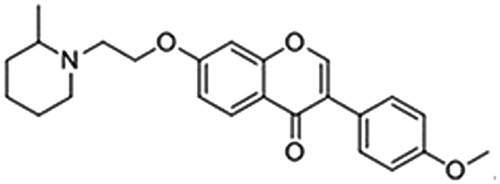
Table 1. Chemical structures of synthesised isoflavone derivatives.
Scheme 1. The general procedure for the synthesis of compounds 1–20. (i): K2CO3, acetone, Br(CH2)nBr, 60 °C; (ii): RH (amines), K2CO3, DMF/Acetonitrile, 100 °C, 3 h; (iii): 40% HBr, 120 °C, 3 h; (iv): piperidine, THF r.t. 10 h; (v): formononetin, K2CO3, acetone; (vi) K2CO3, 3-bromopropionic acid, acetone, 60 °C, 2 days; (vii) DMF, N, N-diisopropylethylamine, piperidine, 12 h, r.t.
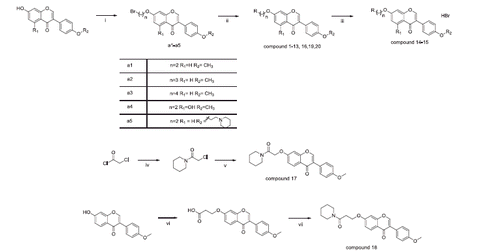
Table 2. In vitro inhibition of AChE and BuChE for compounds 1–20.
Figure 3. (a) 2D schematic diagram of potential interactions between compound 16 and AChE. (b) The predicted binding mode of compound 16 with AChE.
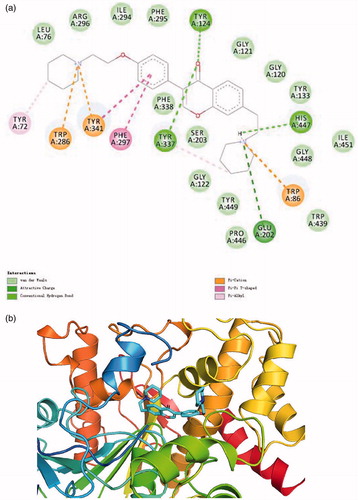
Figure 4. (a) 2D schematic diagram of potential interactions between compound 16 and BuChE. (b) The predicted binding mode of compound 16 with BuChE.
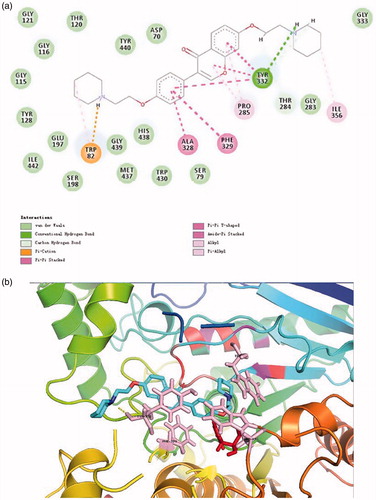
Table 3. Predicted pharmacokinetic properties of compounds 1, 3, 14, 15 and 16.