Figures & data
Figure 1. Judicious design of target 3-phenylthiazolo[3,2-a]benzimidazoles (Series 2) based on cyclisation of disclosed compounds 2-((benimidazol-2-yl)thio)-1-arylethan-1-ones (Series 1).
![Figure 1. Judicious design of target 3-phenylthiazolo[3,2-a]benzimidazoles (Series 2) based on cyclisation of disclosed compounds 2-((benimidazol-2-yl)thio)-1-arylethan-1-ones (Series 1).](/cms/asset/5fc4cbb1-e1d9-4fba-a424-9ef30569d25a/ienz_a_1347166_f0001_c.jpg)
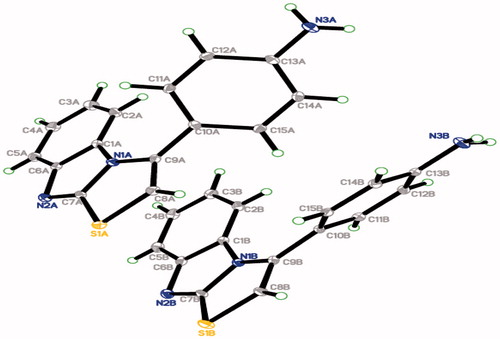
Table 1. Crystallographic data and refinements for compound 4d.
Table 2. Hydrogen-bond geometry (Å, °) of 4d.
Table 3. In vitro anti-proliferative activity of compounds 4a–d against colon HT-29 and breast MDA-MB-468 cancer cell lines.
Table 4. Inhibition (%) of cell surface expression of CD133 on HT-29 cancer cells at 10 μM.

![Scheme 1. Synthesis of target 3-phenylthiazolo[3,2-a]benzimidazoles 4a–d.](/cms/asset/c8e9f737-342c-43e0-8dc4-e797641cd8d5/ienz_a_1347166_sch0001.jpg)