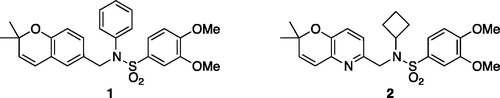
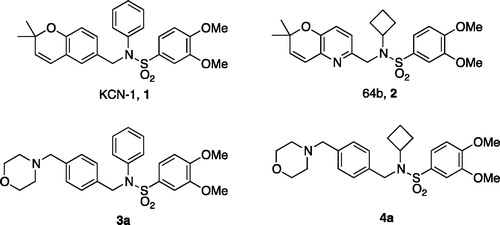
Figures & data
Figure 2. Classes of analogs. (A) Class A, morpholinomethylphenyl in ortho, meta, or para positions, or morpholinophenyl in para position; (B) Class B, morpholinomethylphenyl in ortho, meta, or para positions, or morpholinophenyl in para position; (C) Class C, n = 2 or 3; (D) Class D.
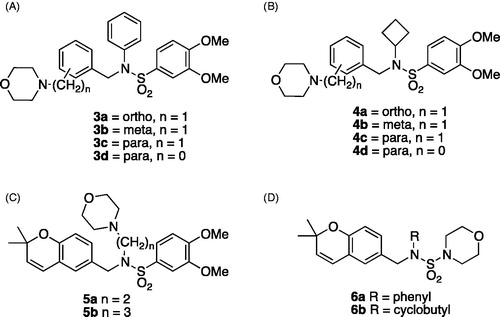
Table 1. Structures, HRE-luciferase reporter inhibitory activity, cLog D, and cLog S of analogs.
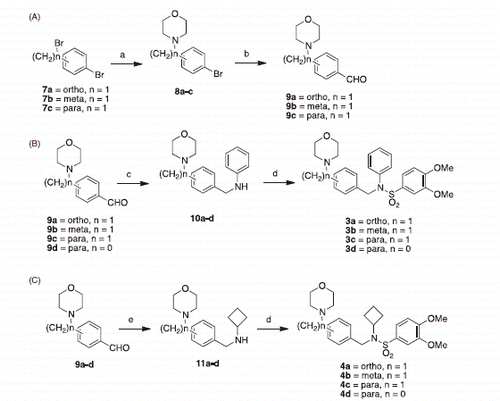
Scheme 1. Synthesis of Class A & B compounds. (A) Synthesis of precursors. (B) Synthesis of Class A. (C) Synthesis of Class B. Reagents and conditions: (a) morpholine, K2CO3, ACN, room temperature, overnight; (b) BuLi, DMF, THF, −78 °C, 1 h; (c) aniline, InCl3, NaBH4, ACN, 20 min; (d) 3,4-dimethoxybenzenesulfonyl chloride, K2CO3, DCM, overnight; (e) cyclobutylamine, NaBH4, MeOH, overnight.
Scheme 2. Synthesis of Class C compounds. Reagents and conditions: (a) amine, NaBH4, MeOH, overnight; (b) 3,4-dimethoxybenzenesulfonyl chloride, K2CO3, DCM, overnight.
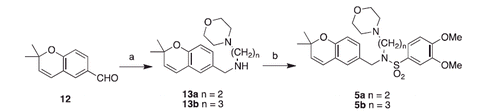
Scheme 3. Synthesis of Class D compounds. Reagents and conditions: (a) aniline, InCl3, NaBH4, ACN, 20 min or cyclobutylamine, NaBH4, MeOH, overnight; (b) 4-morpholinosulfonyl chloride, pyridine, DCE, reflux 2 days.
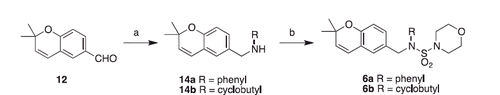
Table 2. Measured solubility of selected compounds.