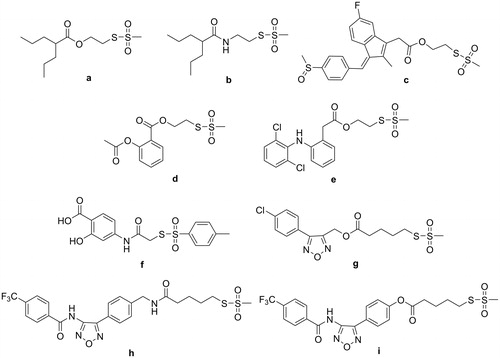
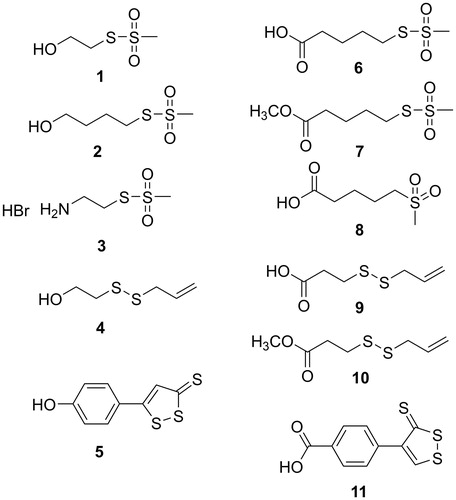
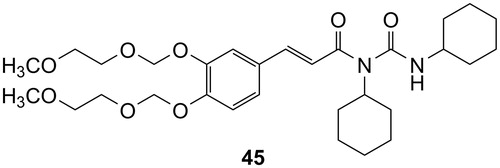
Figures & data
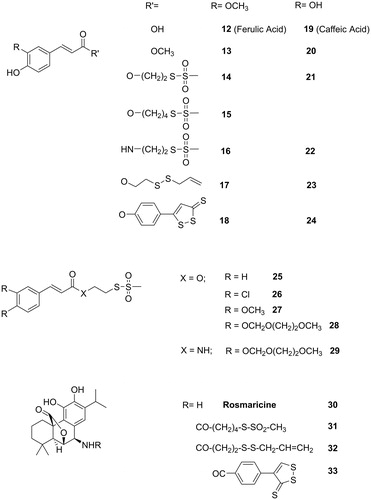
Figure 3. Structures of the investigated derivatives of ferulic, caffeic, and other cinnamic acids and of rosmaricine.
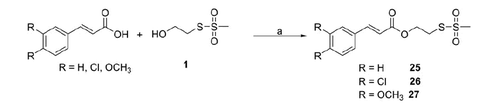
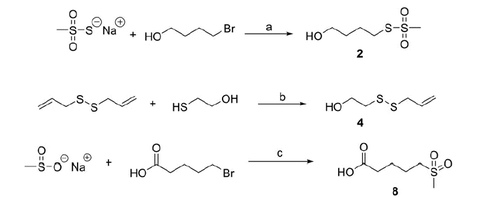
Scheme 1. Reagents and conditions: (a) DMF, N2, 60 °C, 5 h; (b) DMSO, N2, 60 °C, 5 h; (c) dry DMF, 60 °C, 5 h.
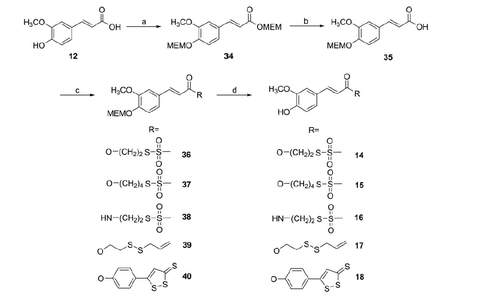
Scheme 2. Reagents and conditions: (a) MEM-Cl, DIPEA, anh. CH2Cl2, 5 h, 0 °C to r.t.; (b) LiOH*H2O, THF/H2O (2:1), 24 h, r.t.; (c) RH (1–5), DCC (EDAC for 38), DMAP, anh. CH2Cl2 (CHCl3 for 38), 3–20 h, r.t.; (d) TFA, anh. CH2Cl2, 4–7 h, r.t.
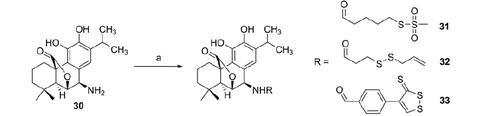
Scheme 3. Reagents and conditions: (a) H2SO4 (cat.), MeOH, reflux, 2 h; (b) NaH, MEM-Cl, anh. THF, 0 °C, 5 h; (c) 5 N NaOH, THF/MeOH (4:1), 40 °C, 3 h; (d) RH (1, 3–5), DCC or EDAC (for 29), DMAP, anh. THF or CH2Cl2 or CHCl3, 4–20 h, r.t.; (e) TFA, anh. CH2Cl2 or CHCl3, 5–8 h, r.t.
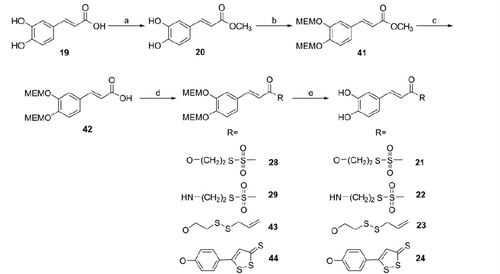
Table 1. Biological activities of sulfurated parent compounds (1–6, 9, and 11) and some related compounds (7, 8, and 10).
Table 2. Biological activities of parent compounds (ferulic acid 12, caffeic acid 19, and rosmaricine) and their sulfurated derivatives.