Figures & data
Figure 1. Dose-dependent effect of cysteine on recombinant CN1 activity. Levels of 0.2 mM cysteine and higher, resulted in significantly reduced CN1 activity (n = 8, p < .005).
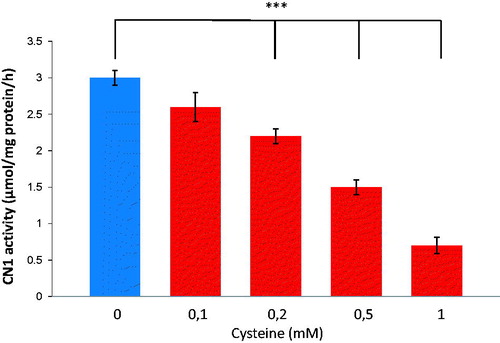
Table 1. Catabolic rate of serum CN1 in the presence of inhibitors (n = 5).
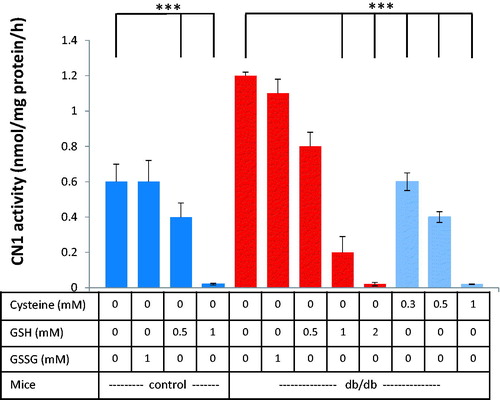
Figure 2. Dose-dependent effect of reduced glutathione (GSH) on renal CN1 activity of db/db mice and controls (at 25 weeks age). Addition of GSH, but not the addition of GSSG, reduced CN1 activity dose-dependently. 0.5 mM GSH significantly lowered CN1 activity for control and control mice (n = 8; p < .005).