Figures & data
Figure 1. Structures of some dithiocarbamates and (1S,4S)-2,5-diazabicyclo[2.2.1]heptanes displaying potent anticancer activity.
![Figure 1. Structures of some dithiocarbamates and (1S,4S)-2,5-diazabicyclo[2.2.1]heptanes displaying potent anticancer activity.](/cms/asset/9dab9ccf-723e-4255-aa75-b8de18357955/ienz_a_1363197_f0001_b.jpg)
Scheme 1. Synthesis of (1S,4S)-2-benzyl-2,5-diazabicyclo[2.2.1]heptane dihydrobromide. Reagents and conditions. (a) TsCl, Na2CO3, H2O, 94%; (b) NaBH4, BF3–Et2O, THF, 85%; (c) TsCl, C5H5N, toluene, 20 h, 83%; (d) PhCH2NH2, toluene, reflux, 96%; (e) HBr 40%, 96%.
![Scheme 1. Synthesis of (1S,4S)-2-benzyl-2,5-diazabicyclo[2.2.1]heptane dihydrobromide. Reagents and conditions. (a) TsCl, Na2CO3, H2O, 94%; (b) NaBH4, BF3–Et2O, THF, 85%; (c) TsCl, C5H5N, toluene, 20 h, 83%; (d) PhCH2NH2, toluene, reflux, 96%; (e) HBr 40%, 96%.](/cms/asset/db809420-54f7-44bd-a3d2-f3fa97ba8ef5/ienz_a_1363197_sch0001.gif)
Figure 2. Structures of the synthesized (1S,4S)-N-Boc-2,5-diazabicyclo[2.2.1]heptane-dithiocarbamates.
![Figure 2. Structures of the synthesized (1S,4S)-N-Boc-2,5-diazabicyclo[2.2.1]heptane-dithiocarbamates.](/cms/asset/9de34a29-2a64-4c64-97da-346bd1a8b65e/ienz_a_1363197_f0002_b.jpg)
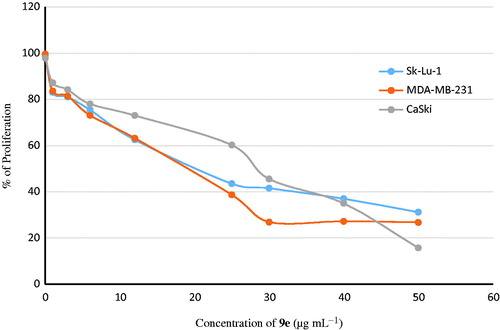
Figure 4. Necrotic effect of 9e (at the IC50 values) on both the tumour and lymphocytes cell lines by LDH leakage assay.
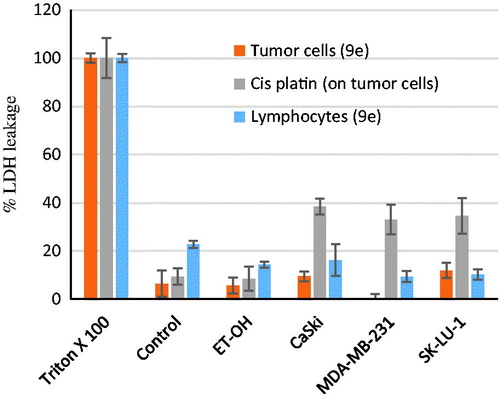
Figure 5. Compound 9e induced apoptotic death. Immunodetection of active caspase-3 by compound 9e on CaSki (A), MDA-MB-231 (B) and SK-Lu-1cultures (C).
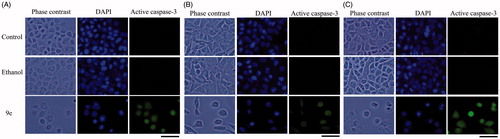
Figure 6. Effect of the compound 9e on lymphocyte proliferation by CFSE-labelling assay [at the concentrations of 18 (left) and 20 (right) µg mL−1].
![Figure 6. Effect of the compound 9e on lymphocyte proliferation by CFSE-labelling assay [at the concentrations of 18 (left) and 20 (right) µg mL−1].](/cms/asset/ca301556-bfee-4304-b80e-914b459f2380/ienz_a_1363197_f0006_c.jpg)
Table 1. Experimental data of the compounds 9a–9g.
Table 2. Spectral data of the compounds 9a–9g.
Table 3. Antiproliferative activities of the synthesized (1S,4S)-N-Boc-2,5-diazabicyclo[2.2.1]heptane-dithiocarbamates (9a–9g).
Table 4. In silico prediction of physicochemical pharmacokinetic propertiesCitation24.

![Scheme 2. Synthesis of (1S,4S)-tert-butyl 2,5-diazabicyclo[2.2.1]heptane-2-carboxylate.](/cms/asset/f5e8bdfc-dae1-4132-8f79-c44f3ea3b1af/ienz_a_1363197_sch0002.gif)
![Scheme 3. One-pot synthesis of (1S,4S)-N-Boc-2,5-diazabicyclo[2.2.1]heptane-dithiocarbamates.](/cms/asset/71cdd8a0-1834-4540-b528-2d569a1768fc/ienz_a_1363197_sch0003.gif)