Figures & data
Figure 1. Chemical structures of apigenin (1), luteolin (2), chrysoeriol (3), quercetin (4), 8α-(2-methylacryloyloxy)-hirsutinolide-13-O-acetate (5), 8α-tigloyloxyhirsutinolide-13-O-acetate (6), 8α-(4-hydroxymethacryloyloxy)-hirsutinolide-13-O-acetate (7), 8α-(4-hydroxytigloyloxy)-hirsutinolide-13-O-acetate (8), 2-(penta-1,3-diyn-1-yl)-5–(4-acetoxy-3-hydroxybuta-1-yn-1-yl) thiophene (9), 2-(prop-1-inyl)-5–(6-acetoxy-5-hydroxyhexa-1, 3-diinyl) thiophene (10), 2-(prop-1-inyl)-5–(5, 6-dihydroxyhexa-1,3-diinyl) thiophene (11). Chemical structure was produced using ChemDraw Professional 8.
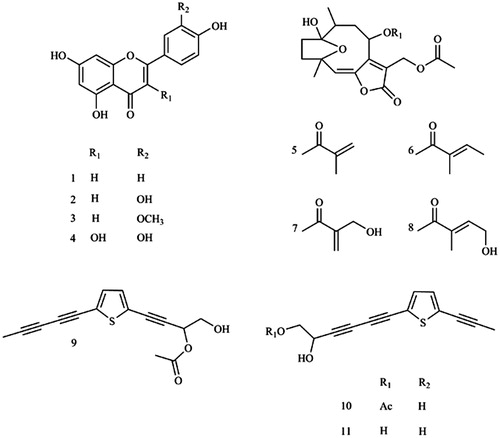
Table 1. IC50 values of extracts and compounds from V. cinerea and P. indica against CYP2A6 and CYP2A13
Table 2. Kinetics values and mode of inhibition of purified compounds from V. cinerea and P. indica against CYP2A6 and CYP2A13.
Table 3. Effects of various trapping agents and dialysis of purified compounds from V. cinerea and P. indica on CYP2A6 and CYP2A13
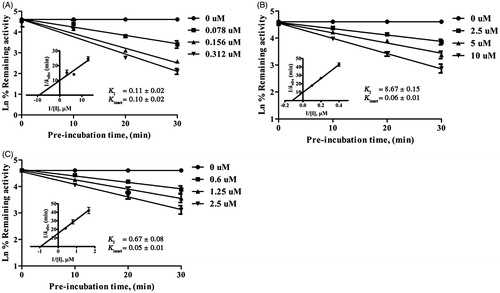
Figure 2. Time- and concentration-dependent inactivation and kinetics of inhibition of CYP2A6-mediated coumarin 7-hydroxylation by 2-(prop-1-inyl)-5–(5, 6-dihydroxyhexa-1,3-diinyl) thiophene 11 (A), and of CYP2A13-mediated coumarin 7-hydroxylation by 8α-(4-hydroxymethacryloyloxy)-hirsutinolide-13-O-acetate 7 (B), and 2-(prop-1-inyl)-5–(5, 6-dihydroxyhexa-1,3-diinyl) thiophene 11 (C). Data are represented as mean ± SD of triplicate experiments.