Figures & data
Figure 3. Scheme of synthesis of target compounds, 3a–e, 3′a–n, 3″a–e. Reagents and conditions: (a) Oxone, NaHCO3/CH3CN/H2O, rt; (b) RCHO, 40–50 °C, 8–12 h, CH2Cl2, PTSA.
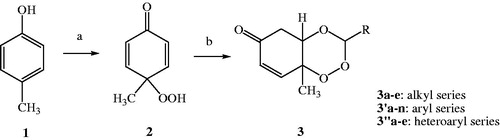
Table 1. Physicochemical data of synthesised compounds.
Figure 5. Photographs showing in vitro antimalarial activity of the most active compound, 3′l against (a) CQ-sensitive 3D7 and (b) CQ-resistant RKL9 strains of P. falciparum.
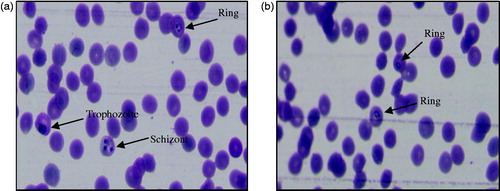
Table 2. In vitro antimalarial activity data.
Figure 6. Structural requirements and effect of substitutions on antimalarial activity of 1,2,4-trioxane derivatives.
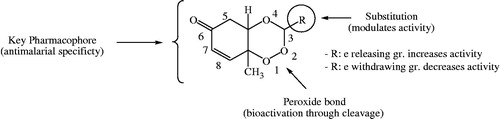
Figure 7. Hypothetical mode(s) of antimalarial action of 1,2,4-trioxane derivatives. (1) Inhibition of haemoglobin degrading enzyme in food vacuole of parasite, (2) direct lethal action to parasite by LPO process. Pf HDE: P. falciparum haemoglobin degrading enzyme; LPO: lipid peroxidation.
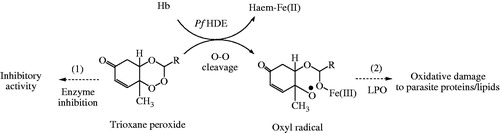
Figure 8. (a) Optimised co-crystal structure of falcipain 2 (chain A)-E-64 and (b) receptor grid for docking.
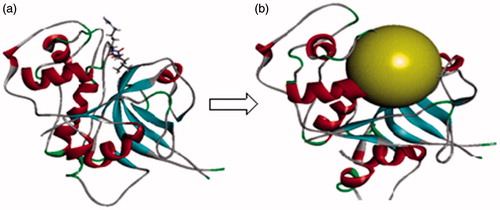
Figure 9. (a) Redocked conformer (pose) of E-64 in the active site of the protein falcipain 2 (left) and 2D representation of the binding interaction (right); (b, c) binding mode (left) and 2D receptor–ligand interaction diagram (right) of compounds, 3′l and 3″d at binding pocket of falcipain 2 (left), respectively.
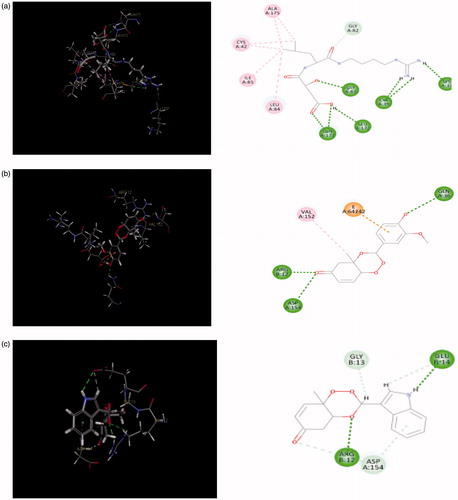
Table 3. LibDock scores, no. of H-bonds and H-bond energies.
Table 4. Details of hydrogen bonding between five most active ligands and receptor molecule.
Figure 10. Graphs showing correlation between in vitro antimalarial activity (pIC50) and LibDock scores for five most potent compounds.
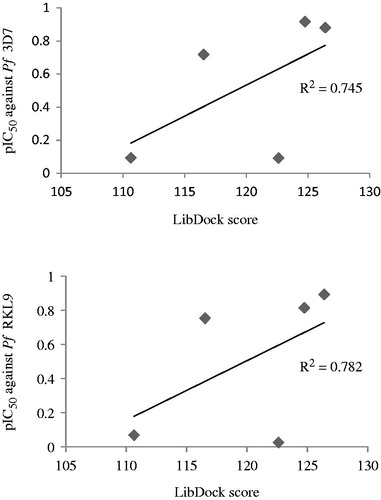
Table 5. Calculated molecular properties and drug-likeness parameters.
Table 6. Theoretical ADMET parameters.