Figures & data
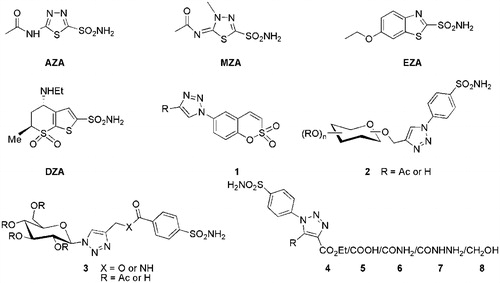
Figure 1. Some clinically used sulphonamide based drugs and 1,2,3-triazole ring containing CA inhibitors.
Scheme 1. Synthetic pathway to the sulphonamides 4a–4d, 5a–5d, 6a–6d, 7a–7d, and 8a–8d. Reagents and conditions: (i) HCl, NaNO2, H2O, 0 °C, 15 min; (ii) NaN3, 0 °C, 30 min; (iii) Piperidine, DMSO, 70 °C, 4 h; (iv) NaOH, reflux, 3 h then H3O+; (v) NH3 solution, stir, 22 h; (vi) NH2NH2.H2O, EtOH, reflux, 10–12 h; (vii) LiAlH4, dry THF, reflux, 2 h then H3O+.
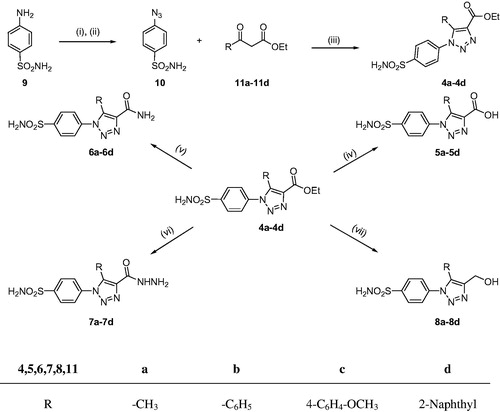
Table 1. Inhibitory potency data for compounds 4a–4d, 5a–5d, 6a–6d, 7a–7d, and 8a–8d against isozymes hCAI, hCA II, hCA IV, and hCA IX.
