Figures & data
Figure 1. The hydrolyze reaction of acetylcholine in the presence of acetylcholinesterase enzyme (AChE).
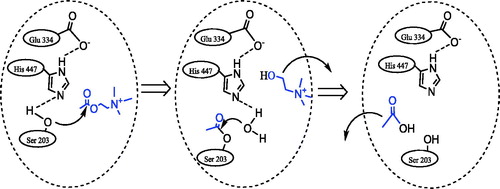
Table 1. Physico-chemical characteristics of aminomethyl derivatives of benzothiazol- and benzoxazolthiones
Table 2. Physico-chemical characteristics of the alkoxymethyl derivatives of benzoxazolthione and 2-aminothiazoles.
Table 3. AChE, human carbonic anhydrase I, and II isoforms (hCA I, and II) AChE and BChE enzymes inhibition effects of aminomethyl and alkoxymethyl derivatives (1–17) and proportion of AChE to BChE enzymes.
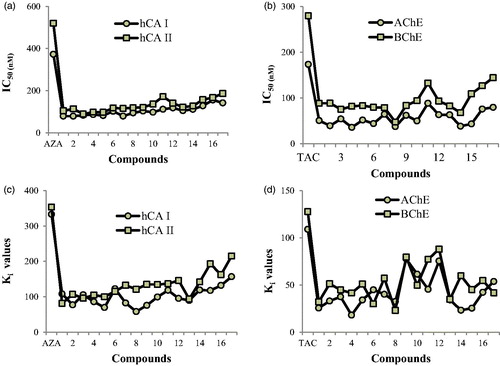
Figure 2. (a) IC50 values of aminomethyl and alkoxymethyl derivatives for hCA I, and II isoenzymes. (b) IC50 values of aminomethyl and alkoxymethyl derivatives for AChE and BChE enzymes. (c) Ki values of aminomethyl and alkoxymethyl derivatives for hCA I, and II isoenzymes. (d) Ki values of aminomethyl and alkoxymethyl derivatives for AChE and BChE enzymes.