Figures & data
Figure 2. (A) Inhibitory effect of compound (1) and positive control (NaVO4) on PTP1B activity. (B) Determination of the reversible inhibitory mechanism of compound 1 on PTP1B. Data represent the results of three independent experiments performed in triplicates for each sample.
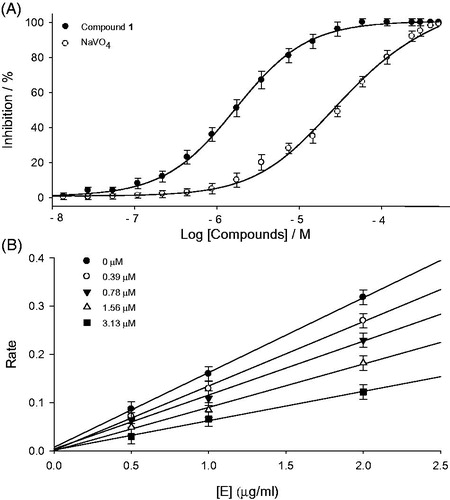
Figure 3. (A–D) Kinetic assays of PTP1B inhibition, caused by compounds 1 and 6. (A and B) Lineweaver–Burk plots were constructed for the inhibition of PTP1B. The plots are expressed as 1/velocity (1/V) versus 1/substrate (1/[S]) with or without inhibitors. Insets (I) and (II) represents the secondary plots of the slopes and the intercepts of the straight lines versus concentrations of compound 1 and 6. (C and D) Dixon plots for inhibition of PTP1B by compound 1 and 6, respectively.
![Figure 3. (A–D) Kinetic assays of PTP1B inhibition, caused by compounds 1 and 6. (A and B) Lineweaver–Burk plots were constructed for the inhibition of PTP1B. The plots are expressed as 1/velocity (1/V) versus 1/substrate (1/[S]) with or without inhibitors. Insets (I) and (II) represents the secondary plots of the slopes and the intercepts of the straight lines versus concentrations of compound 1 and 6. (C and D) Dixon plots for inhibition of PTP1B by compound 1 and 6, respectively.](/cms/asset/f2855da4-1974-4861-bfbd-c76d14042b21/ienz_a_1368502_f0003_b.jpg)
Table 1. Inhibitory activities on PTP1B and α-glucosidase of isolated compounds.
Figure 4. (A) Inhibitory effects of compounds (1–8) on α-glucosidase activity. (B and C) Linweaver–Burk plots of compounds 4 and 8 for the inhibition of α-glucosidase catalyzed hydrolysis of p-nitrophenyl glucopyranoside. (D and E) Dixon plots of inhibition of α-glucosidase by compounds 4 and 8, respectively, which were used for determination of Ki values.
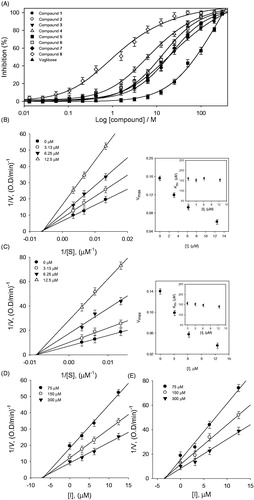
Table 2. Effect of different concentrations of compound 1 on Vmax, Km, and the Kik to Kiv ratio using PTP1B and α-glucosidase.