Figures & data
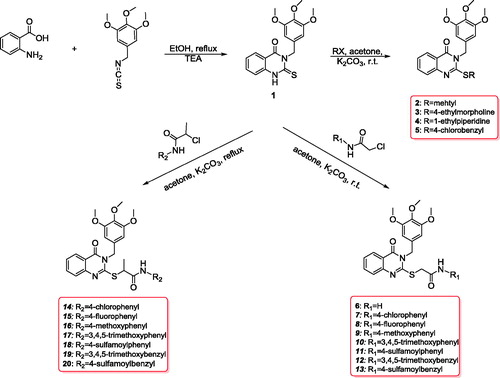
Figure 1. Structures of erlotinib, gefitinib, reported compounds A–D, and designed quinazoline derivatives E–H as antitumour agents.
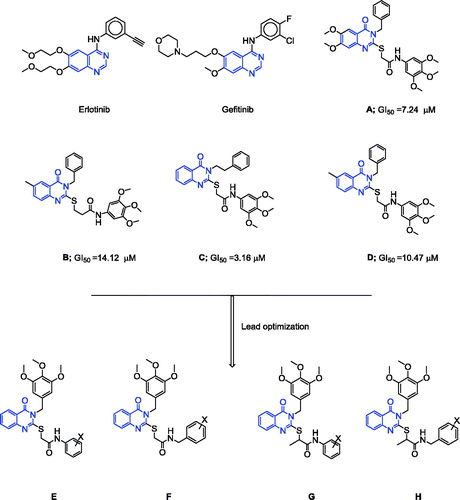
Table 1. Percentage growth inhibition (GI %) of in vitro subpanel tumour cell lines at 10 µM concentration.
Table 2. Median growth inhibitory (GI50, μM), total growth inhibitory (TGI, μM), and median lethal (LC50, μM) concentrations of compounds 7 and 19 on in vitro subpanel tumour cell lines.
Table 3. GI50 values (μM) of compounds 7 and 19 compared with those of erlotinib, gefitinib, and 5-FU on in vitro subpanel tumour cell lines.
Figure 2. Three-dimensional (3D) interactions of erlotinib (upper panel), compounds 19 (middle panel) and 7 (lower panel) with the receptor pocket of EGFR kinase. Hydrogen bonds are shown with a green line.
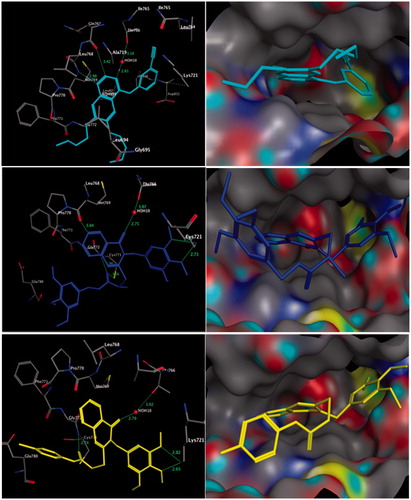
Table 4. Results of the docking of compounds 7 and 19 into EGFR (pdb: 1m17), in comparison to the co-crystallised ligand (erlotinib).