Figures & data
Figure 1. The structure of the reported compound 1 having anti-TB activityCitation8.
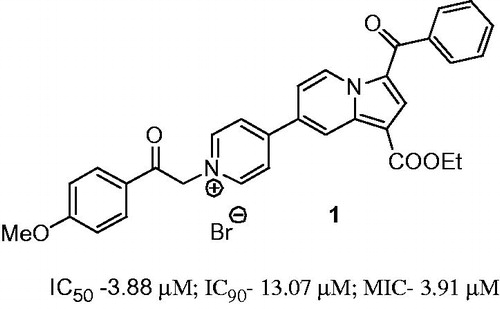
Table 1. Results of antimycobacterial activity of compounds 6 against M. tuberculosis H37Rv grown under aerobic conditions.
Table 2. Revaluation of antimycobacterial activity of compounds 6a, 6c, 6d, 6h and 6i against M. tuberculosis H37Rv grown under aerobic conditions.
Table 3. The bactericidal activity (MBC) of compounds 6a, 6c, 6d, 6h and 6i.
Table 4. Results of antimycobacterial activity of compounds 6a, 6c, 6d, 6h and 6i against M. tuberculosis H37Rv under low oxygen.
Table 5. MIC, IC50 and IC90 of compounds 6 against M. tuberculosis resistant at different treatments and non-tuberculous mycobacteria.
Table 6. Results of cytotoxicity evaluation.
Table 7. Results of plasma protein binding assay for compound 6i.
Table 8. Permeability evaluation of compound 6i.
Table 9. Cytochrome P450 inhibition results.
Table 10. In vitro microsomal stability assay.