Figures & data
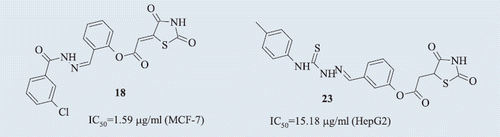
Figure 1. Synthesis of 2-(2,4-dioxothiazolidin-5-yl)acetyl chloride and 2-(2,4-dioxothiazolidin-5-ylidene)acetyl chloride. Reagent and conditions: (i) HCl, reflux; (ii) Br2, CH3COOH, reflux; (iii) SOCl2, DMF, 1,4-dioxane, reflux 1 h.
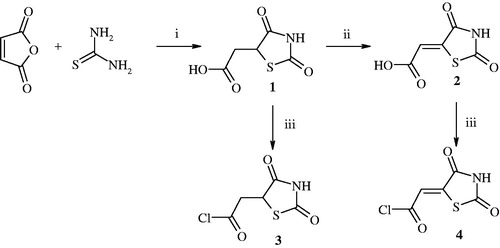
Figure 2. Synthesis of target compounds (12–28) 2-(2,4-dioxothiazolidin-5-yl)acetic and 2-(2,4-dioxothiazolidin-5-ylidene)acetic acid derivatives. Reagent and conditions: (i) pyridine, 1,4-dioxane, rt, after 2 h acidified of solution of hydrochloric acid; (ii) 3-chlorobenzhydrazide or 2,4-dichlorobenzhydrazide, anhydrous ethanol, reflux; (iii) corresponding 4-substituted thiosemicarbazide derivatives, anhydrous ethanol, reflux.
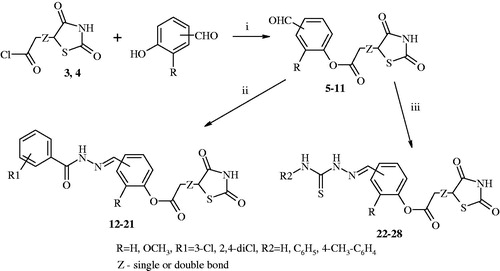
Table 1. Antiproliferative activity of novel thiazolidine-2,4-dione derivatives and irinotecan used as a reference substance.
Table 2. Newly synthesised compounds’ influence on the Gram-positive bacterial strains growth – MIC (μg/ml) values.
