Figures & data
Figure 1. Chemical structures of clinically used CAIs and pyridinium containing sulfonamides 1 and 2.
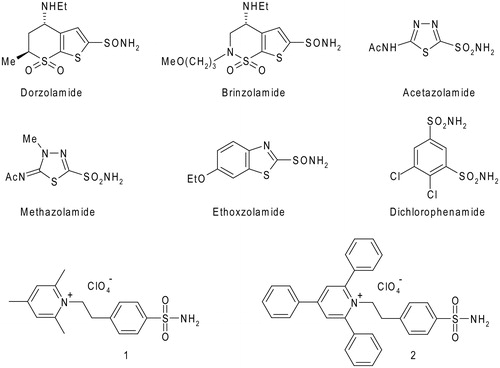
Table 1. Inhibition of isozymes hCA I, hCA II, hCA IV, hCA IX, and hCA XII with the pyridinium salts 1, 2, and the standard, clinically used sulfonamide CAIs.
Table 2. Data collection and refinement statistics for the hCA II/2 complex.
Figure 2. Active site region of the hCA II/2 adduct, showing σA-weighted |2Fo − Fc| simulated annealing omit map (contoured at 1.0σ) relative to the inhibitor molecule. Active site Zn2+ coordination (red continuous lines) and hydrogen bonds (red dotted lines) are also reported.
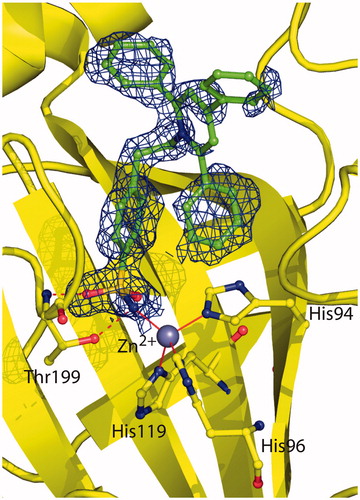
Figure 3. Structural superposition between 1 (cyan, PDB code 1ZE8)Citation41 and 2 (green) when bound to hCA II active site.
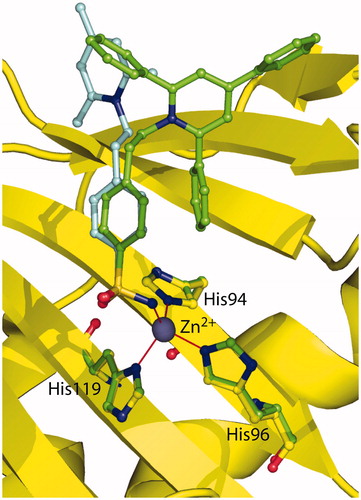
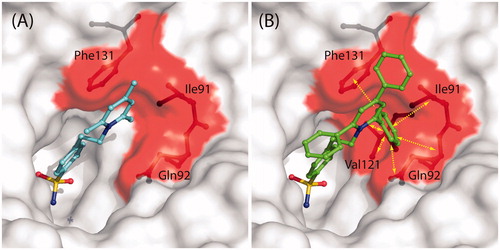
Figure 4. (A) Solvent accessible surface of hCA II/1 active siteCitation41. Residues delimiting the hydrophobic pocket, where the trimethyl-pyridinium ring is located, are highlighted in red (Ile91, Gln92, Phe131). Compound 1 and residues Ile91, Gln92, and Phe131 are represented as ball-and-stick. (B) Solvent accessible surface of hCA II active site. Colour code is as in (A). The hypothetical conformation that compound 2 would have had if its pyridinium ring was positioned in the same hydrophobic pocket of 1 is shown. Yellow arrows indicate protein residues which clashes with inhibitor (Ile91, Gln92, Val121, Phe131).