Figures & data
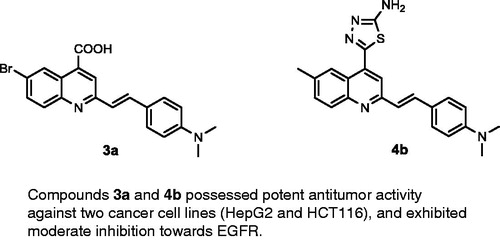
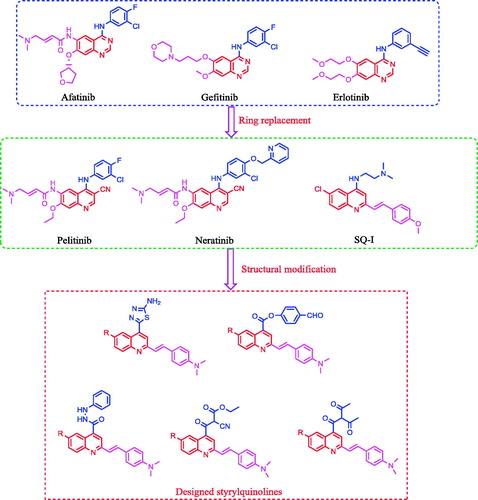
Figure 1. Reported EGFR inhibitors and antitumour agents, and design of the newly synthesized 2-styrylquinolines.
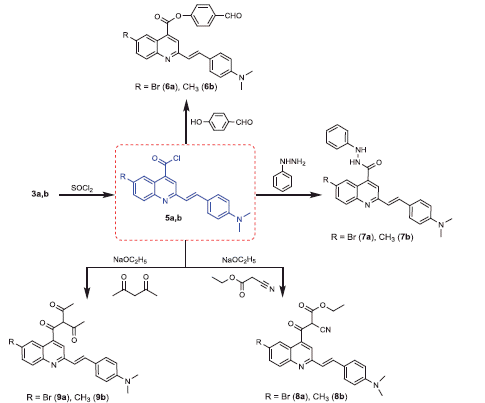
Scheme 1. Synthesis of the designed 2-styryl-4-quinoline carboxylic acids, and 1,3,4-thiadiazoles 3a,b and 4a,b.
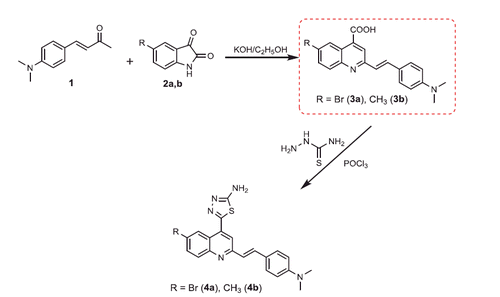
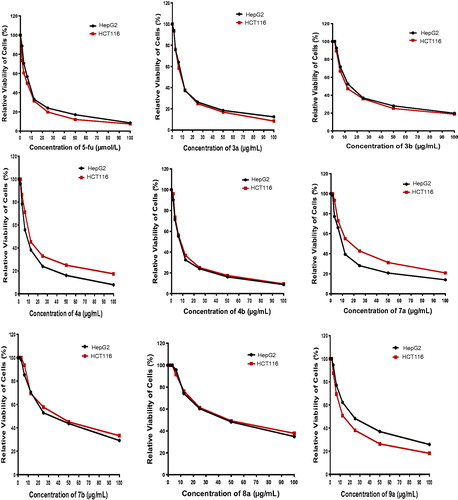
Table 1. In vitro antitumour activity of the tested compounds.
Table 2. IC50 values of the designed compounds toward EGFR kinase and docking interaction energy.
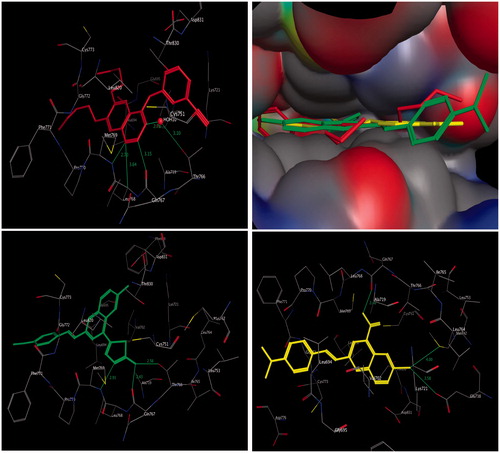
Figure 3. Three-dimensional interactions of erlotinib (upper left panel), compounds 4b (lower left panel), and 3a (lower right panel) with the receptor pocket of EGFR kinase. Hydrogen bonds are shown as green line. Upper right panel shows superimposition of compounds 4b (green coloured) and 3a (yellow coloured) on erlotinib (red coloured) inside the pockets of the active site.