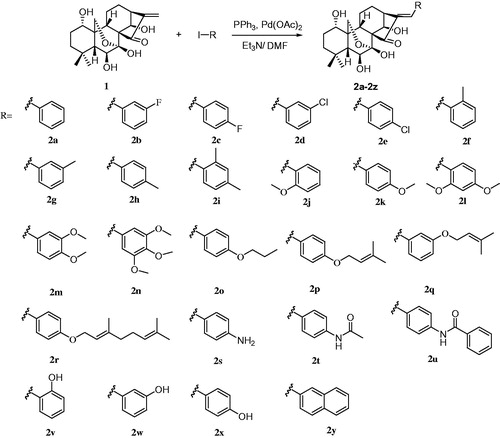
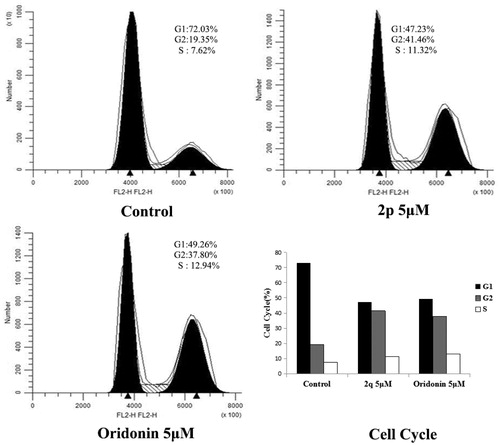
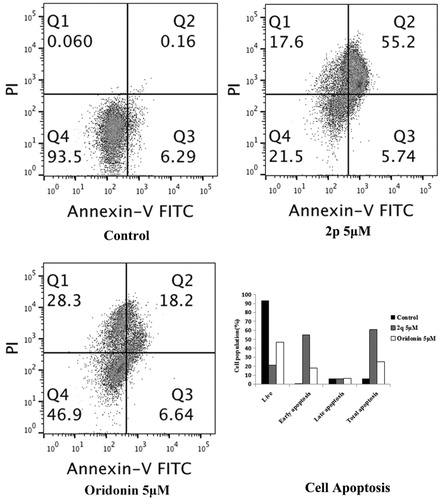
Figures & data
Figure 1. The structure of oridonin, costunolide, compound 3, 4, 5 and the design of target compound 2a–2y.
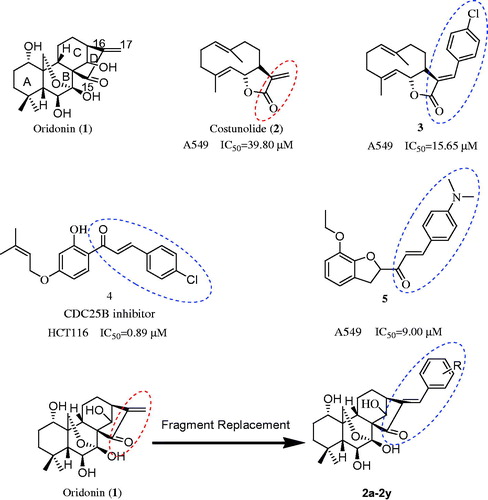
Table 1. Antiproliferative efficacy of oridonin derivatives of 2a–2y in six human cancer cell linesTable Footnoteb.
Table 2. In vitro antiproliferative activities of compounds 2l and 2p against normal cell line (L02).