Figures & data
Scheme 1. General synthetic route to NO-donor compounds 3a–3e and 7a–7c. Reagents and conditions: (a) dibromo alkane, K2CO3, acetone, reflux, 6 h; (b) AgNO3, acetonitrile, 80 °C, 2 h; (c) ethyl bromoacetate, K2CO3, acetone; (d) KOH, methanol; (e) dibromo alkane, Et3N, acetone, reflux, 24 h.
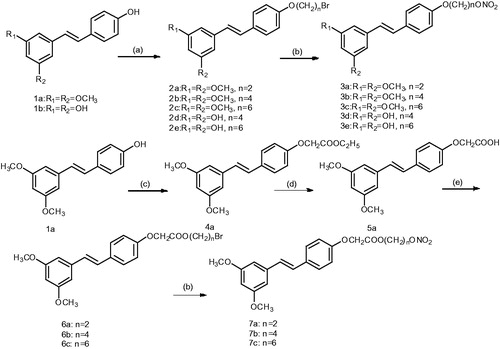
Table 1. Biological evaluation in vitro.
Figure 1. Acute effect of compounds 3c, 3e, and 7c on blood glucose levels in STZ-diabetics mice. Each value is the mean ± SEM for six mice in each group. *p < .05 significantly different ANOVA followed by Dunnett’s t-test for comparison with respect to initial levels in each group.
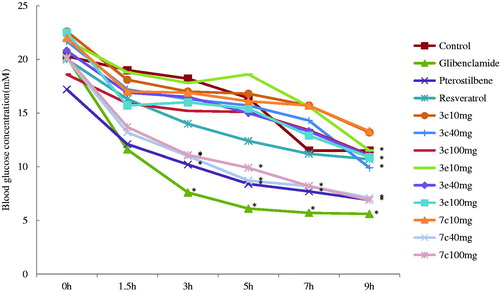
Table 2. Acute effect of compounds 3c, 3e, and 7c on blood glucose levels in STZ-diabetics mice.
Table 3. The amount and order of each reactant of α-glucosidase inhibition test.
