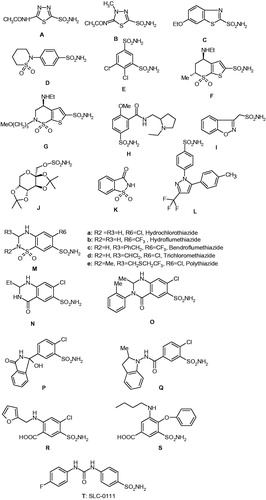
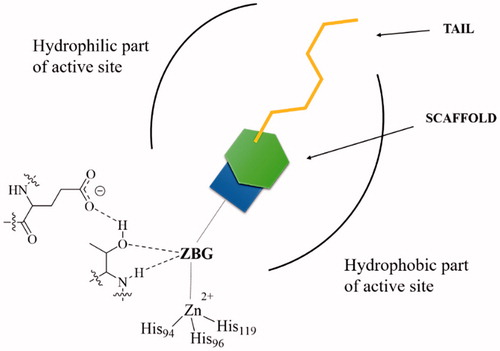
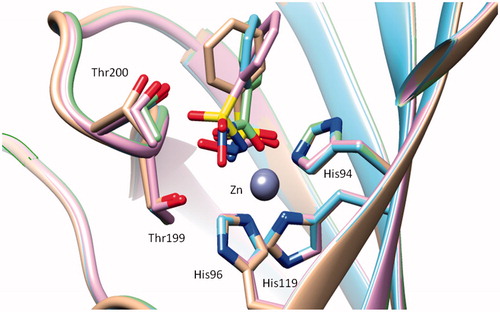
Figures & data
Table 1. Inhibition of CA isozymes I–XV (of human = h, and murine = m origin) with compounds 1–10.
Table 2. hCA I, II, IX, and XII inhibition data with compounds 2, 2a, 2b, 7, and 7a.