Figures & data
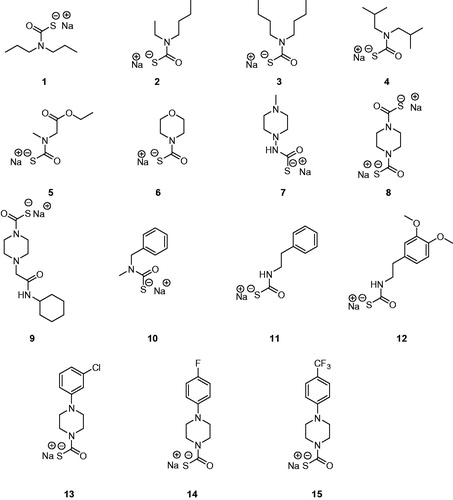
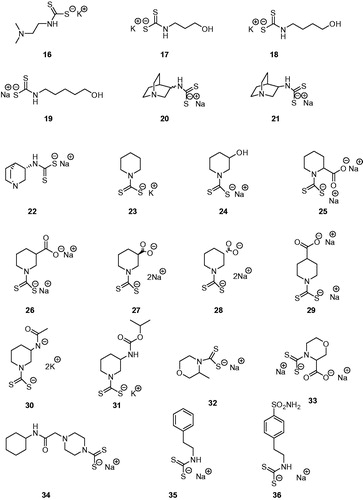
Table 1. TweCAδ, hCA I, and hCA II Inhibition Data with MTCs 1–15, DTCs 16–36, and acetazolamide (AAZ, 5-acetamido-1,3,4-thiadiazole-2-sulphonamide) as standard drug, by a stopped-flow CO2 hydrase assay.