Figures & data
Figure 4. PBrP delays TGF-β-induced cell migration. Confluent monolayers of A549 cells in a 24-well cluster tissue culture plate were scratched and incubated at 37 °C for 20 h in DMEM containing 0.2% of FBS (Control; top). Cell motility was measured using a fully automatic microscope at 10× phase objective. Cell migration was observed by performing time-lapse microscopy and images of all four experimental conditions were captured simultaneously every 20 min. The red lines indicate the starting point of cell migration. Representative micrographs from three experiments are shown in panel (A). Right graphs illustrate of quantitative analyses of cell covering area (mean ± SD) from three independent experiments are shown (B) (% of TGF-β treatment alone); **p < .01. Bar: 10 μm.
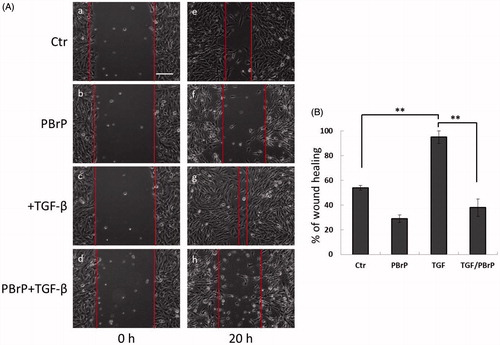
Figure 6. MyoVa depletion enhances TβRII turnover and mitigates TGF-β/Smad signalling. A549 cells were infected with the lentivirus carrying control (Ctr shRNA) or MyoVa shRNA constructs (MyoVa shRNA#1 and MyoVa shRNA#2). Three days post-infection, total protein was extracted and subjected to Western blot using anti-MyoVa, anti-TβRII or anti-β-actin antibodies. (A) Two MyoVa shRNAs abolish MyoVa protein production and reduced the TβRII protein levels. (B) Effects of MyoVa on TGF-β-induced Smad2/3 phosphorylation. A549 cells harbouring MyoVa shRNA#1 or control shRNA were stimulated with 5, 10, 20, 50 and 100 pM of TGF-β for 30 min. The amount of pSmad2/3 obtained from the lysates of cells in the absence (Lanes 8–12) or presence (Lanes 1–7) of MyoVa was analysed through Western blotting using anti-pSmad2/3, anti-Smad2/3 and anti-β-actin were used as loading control. (C) MyoVa depletion inhibits TGF-β-induced fibronectin, PAI-1, and N-cadherin expression. Control and MyoVa-silenced A549 cells were treated with 5, 10, 20, 50 and 100 pM of TGF-β for 48 h. The protein abundance of cells in the absence (Lanes 8–12) or presence (Lanes 1–7) of MyoVa was analysed through Western blotting using appropriate antibodies. Right graphs illustrate quantitative analyses of ECL (mean ± SD) from at least three independent experiments; **p < .01.
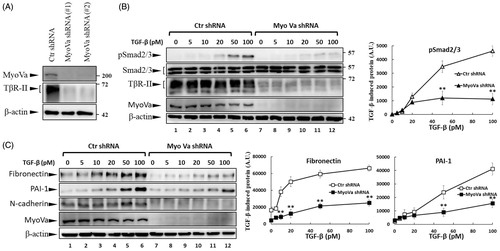
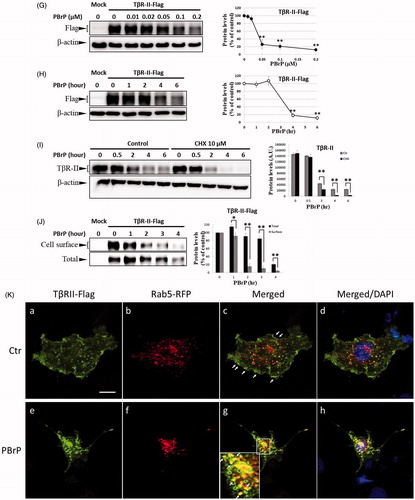
Figure 7. PBrP induces TβRII turnover through the late endosome–lysosome pathway and is impaired by lysosome inhibitors. Mv1Lu cells grown in 0.2% of FBS-containing DMEM were incubated with PBrP (0.5 μM) with chloroquine (100 μM) or NH4Cl (20 mM) (A) for 1, 3 and 6 h or MG132 (20 μM) and carfilzomib (0.5 μM) (B). Subsequently, the cell lysates were subjected to SDS-PAGE, and TβRII expression was analysed through Western blotting and quantified through densitometry. (C) TβRII was enriched in the Rab11-positive endosomal compartment in PBrP-treated cells. Mv1Lu cells were treated with 0.5 μM of PBrP for 1 h in low-serum DMEM. Endogenous TβRII and Rab11 were visualised through immunofluorescence staining using Alexa Fluor 488- and 594-conjugated secondary antibodies, respectively. (D) TβRII was internalised and directed into lysosomes in PBrP-treated cells. HEK293 cells transiently co-expressing TβRII-flag and Lamp-1-GFP were treated with 0.5 μM of PBrP for 2.5 h in low-serum DMEM. After fixation and permeabilisation, TβRII-flag was visualised through immunofluorescence staining using anti-flag antibodies and an Alexa Fluor 594-conjugated secondary antibody. PBrP reduced membrane-associated TβRII-flag (red) and increased co-localisation with the lysosome marker Lamp-1 (green). Bar: 10 μm.
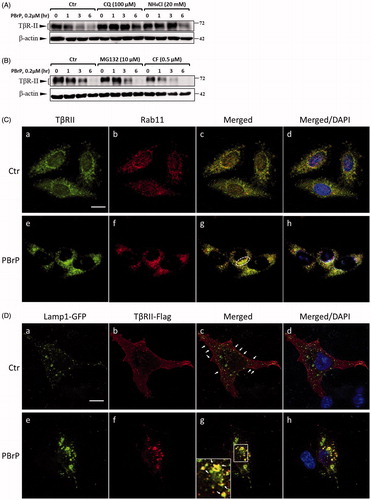
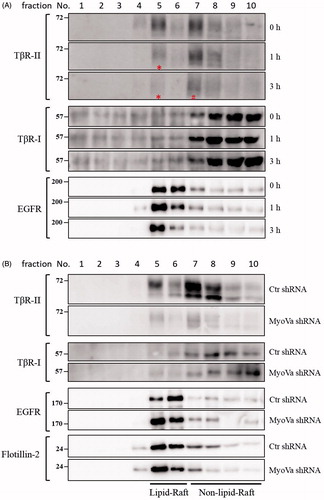
Figure 8. PBrP induces TβRII degradation in lipid rafts. A549 cells or shRNA-silenced A549 cells were left untreated or incubated with 0.5 μM of PBrP for 0, 1 and 3 h in low-serum DMEM and lysed. Subsequently, 10 sucrose density gradient fractions of the lysates were collected through ultracentrifugation, as described in Materials and methods section. Thirty microgram of protein from each fraction was subjected to SDS-PAGE and transferred onto PVDF membranes, and blotted with anti-TβRII, anti-TβRI and EGFR antibodies. Protein samples from various treatments in acrylamide gel strips were synchronously electrotransferred onto the same PVDF membrane and then simultaneously proceeded for Western blotting; therefore, the signals of distinct proteins from various treatments were mutually comparable. (A) PBrP treatment reduced TβRII predominately in raft fractions within 3 h. (B) MyoVa depletion reduced TβRII in raft and non-raft fractions without altering the protein abundance and subcellular compartmentation of TβRI, EGFR or raft marker flotillin-2.