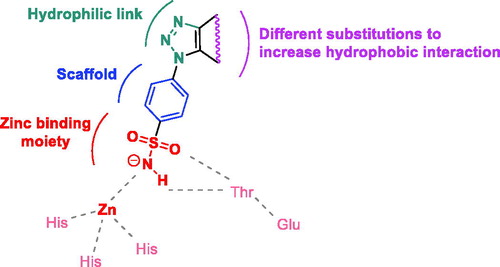
Figures & data
Table 2. Percentage growth inhibition (GI%) of in vitro human tumour cell lines at 10 µM concentration for ten compounds.
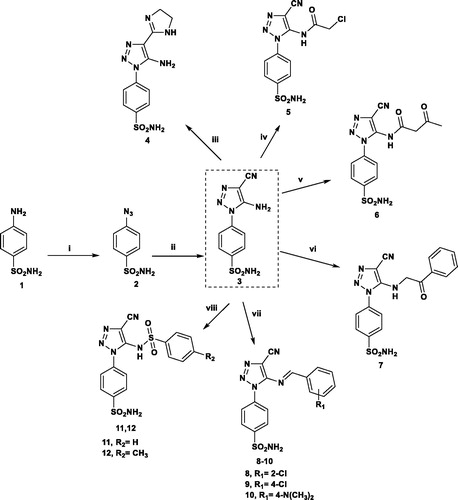
Scheme 1. Reagents and conditions: (i) NaN3/H2SO4/NaNO2/r.t.; (ii) CH2(CN)2/EtONa/EtOH/r.t.; (iii) NH2CH2CH2NH2/CS2/reflux 6 h; (iv) ClCH2COCl/DMF/r.t.; (v) CH3COCH2COOC2H5/reflux 3 h; (vi) PhCOCH2Br/EtOH/reflux 3 h; (vii) Ar-CHO/AcOH/reflux 5 h; (viii) Ar-SO2Cl/pyridine/8 h.
Scheme 2. Reagents and conditions: (i) CNCH2COOC2H5/3 h; (ii) Ar-CHC(CN)2/EtOH/TEA/5 h; (iii) NH2CSNH2/15 min; (iv) PhNCO/DMF/TEA/reflux 18 h; (v) CH3CH2 or Ar-NCS/DMF/TEA/reflux 5 h.
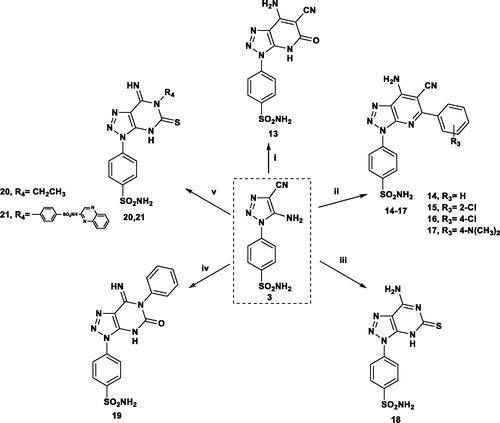
Table 1. Inhibition data of human CA isoforms hCA I, II, IV and IX with compounds 2–21 reported here and the standard sulfonamide inhibitor acetazolamide (AAZ) by a stopped flow CO2 hydrase assay.