Figures & data
Figure 1. hCA I adduct of famotidine (FAM). (a) Overall structure. (b) Active site details, with the Zn(II) ion (gray sphere), its three His ligands and the inhibitor in green.
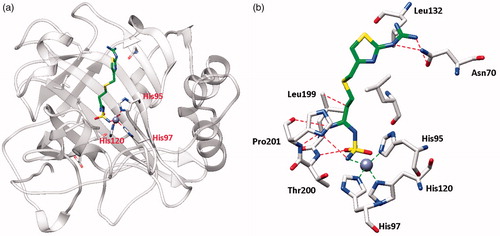
Figure 2. hCA II adduct of famotidine (FAM). (a) Overall structure. (b) Active site details, with the Zn(II) ion (gray sphere), its three His ligands and the inhibitor in green.
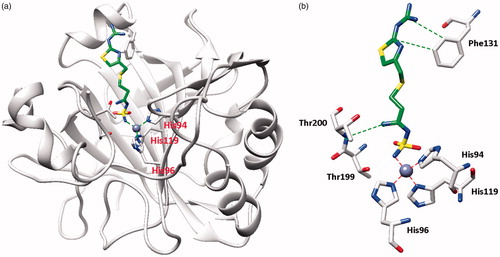
Table 1. Inhibition data against bacterial, diatom, fungal, protozoan, insect and human CAs with famotidine (FAM) and acetazolamide (AAZ) by a stopped flow CO2 hydrase assayCitation22.
