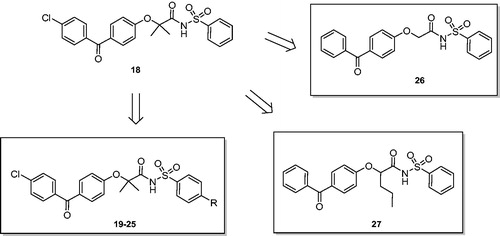
Figures & data
Table 1. Inhibitory activity of derivatives 1–18 and reference compound acetazolamide (AAZ) against four selected hCA isoforms (I, II, IX, and XII) by stopped-flow CO2 hydrase assayCitation31–35.
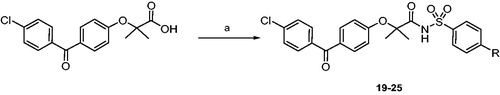
Scheme 1. Reagents and conditions: (a) p-substituted benzenesulphonamide, EDC, DMAP, dry dichloromethane, 0 °C-r.t., 24 h.
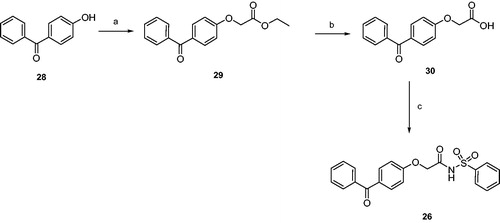
Scheme 2. Reagents and conditions: (a) ethyl 2-bromoacetate, sodium, absolute ethanol, reflux, 20 h; (b) 2N NaOH, THF, r.t., 24 h; (c) benzenesulphonamide, EDC, DMAP, dry dichloromethane, 0 °C-r.t., 24 h.
Scheme 3. Reagents and conditions: (a) ethyl 2-bromovalerate, sodium, absolute ethanol, reflux, 20 h; (b) 2N NaOH, THF, r.t., 24 h; (c) benzenesulphonamide, EDC, DMAP, dry dichloromethane, 0 °C-r.t., 24 h.
Table 2. Inhibitory activity of derivatives 19–27 and reference compound acetazolamide (AAZ) against four selected hCA isoforms (hCA I, II, IX, and XII) by stopped-flow CO2 hydrase assayCitation31,Citation32.
Figure 2. (A) The docked pose of compound 18 (turquoise), (B) compound 21 (purple) and (C) compound 1 (R-isomer; green) in the active site of hCA I (pdb: 3lxe). Hydrogen bonds and interactions to the active site zinc ion are indicated in red dashed lines. Aromatic system – H bonds are indicated in yellow dashed lines.
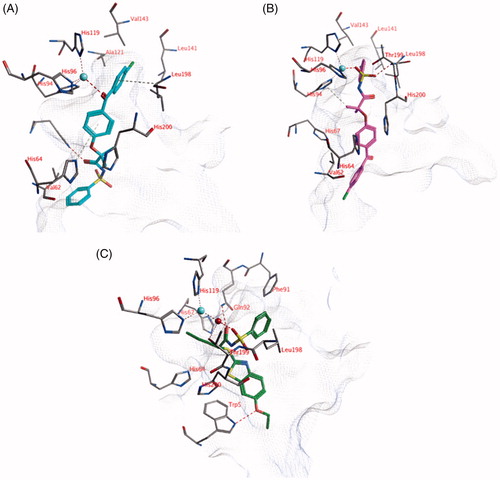
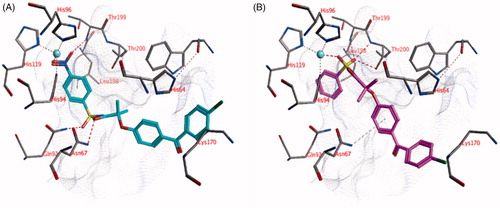
Figure 3. The docked pose of (A) compound 22 (turquoise) and (B) compound 18 (purple) in the active site of hCA II (pdb: 4e3d). Hydrogen bonds and interactions to the active site zinc ion are indicated in red dashed lines. Aromatic system – H bonds are indicated in yellow dashed lines.
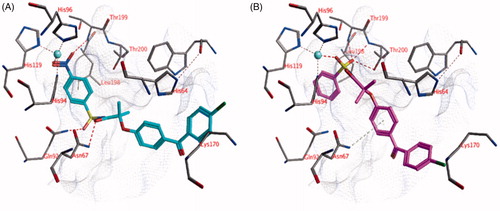
Figure 4. The docked pose of (A) compound 18 (purple) and (B) alternative docked pose of compound 18 (purple) in the active site of hCA IX (pdb: 3iai). Hydrogen bonds and interactions to the active site zinc ion are indicated in red dashed lines. Aromatic system – H bonds are indicated as yellow dashed lines.