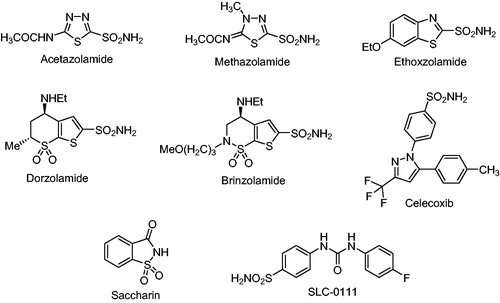
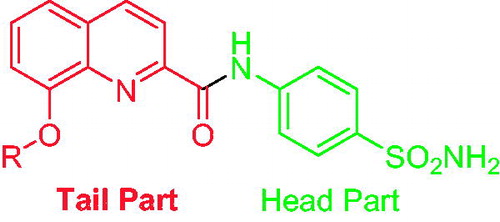
Figures & data
Scheme 1. General synthetic route for the synthesis of 8-substituted quinoline-linked sulfonamide derivatives (5a–h). Reagents and conditions: (i) SeO2, 1,4-dioxane, 110 °C, 12 h, (ii) H2O2, Formic acid, 0 °C, 12 h, (iii) Sulfanilamide, HATU, DIPEA, DMF, 0 °C-rt, 12–15 h, and (iv) R-X, K2CO3, Acetone, r.t., 12–15 h.
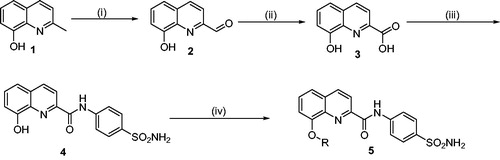
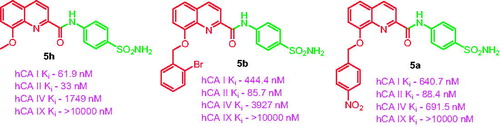
Table 1. CA inhibition data with the synthesised compounds 5a–5h and acetazolamide as standard drug, by a stopped flow CO2 hydrase assay.