Figures & data
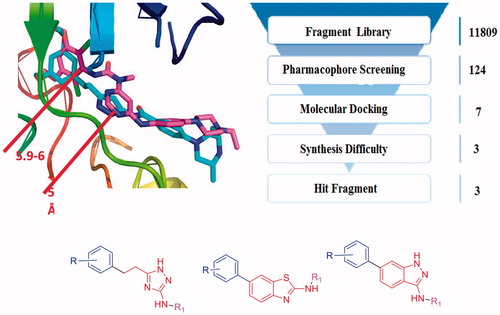
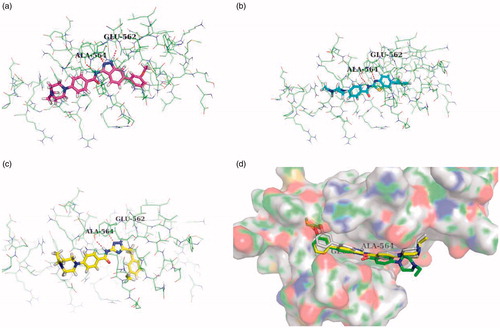
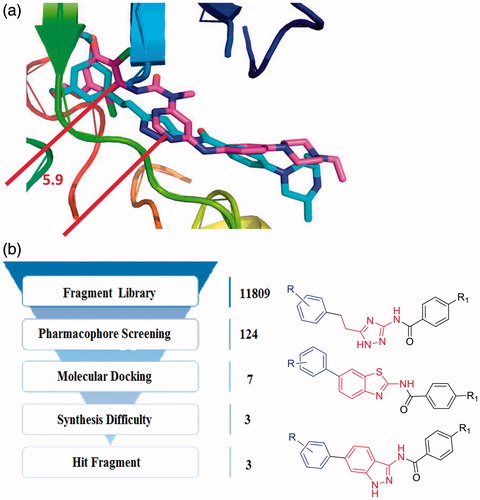
Figure 2. (a) Binding model of AZD4547 and NVP-BGJ398 to the FGFR1 kinase domain (PDB ID: 4V05, 3TTO). (b) The fragment-based virtual screening protocol.
Scheme 1. Synthesis of indazole derivatives. Reagents and conditions: (a) K2CO3, DMSO, 120 °C, 85–90%; (b) NaOH, MeOH, H2O, 80 °C, 93–96%; (c) HATU, K2CO3, DMF, rt, 62–70%; (d) hydrazine hydrate, n-butanol, 120 °C, 86%; (e) Boc2O, DMAP, THF, 89%; (f) NaH, THF, 60 °C, 52–61%; (g) TFA, CH2Cl2, 0 °C, 70%; (h) R-B(OH)2, Cs2CO3, Pd(dppf)Cl2, dioxane, 120 °C, 30–48%.
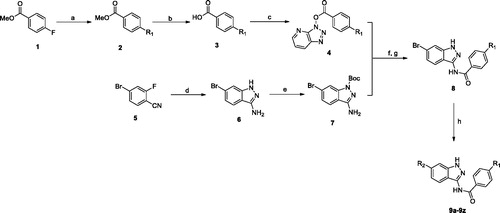
Scheme 2. Synthesis of benzothiazole derivatives. Reagents and conditions: (a) 4, NaH, THF, 60 °C, 51–62%; (b) R-B(OH)2, Cs2CO3, Pd(dppf)Cl2, dioxane, 120 °C, 47–53%.
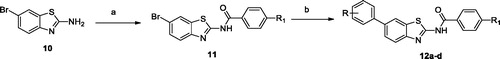
Scheme 3. Synthesis of 1H-1,2,4-triazole derivatives. Reagents and conditions: (a) propanedioic acid, pyridine, piperidine, 105 °C, 77–82%; (b) Pd/C, H2, EtOH, 88–93%; (c) SO2Cl2, reflux; (d) amino guanidine hydrochloride, 140 °C, 49–53%; (f) 4, NaH, THF, 60 °C, 54–62%; (g) TFA, CH2Cl2, 0 °C, 70%.
Table 1. Structures and activities of various derivatives 9a-d, 12a-d, and 18a-c.
Table 2. Structures and activities of indazole derivatives 9e–t.
Table 3. Structures and activities of indazole derivatives 9 u–z.
Table 4. Selectivity of 9 u in a panel of kinases.