Figures & data
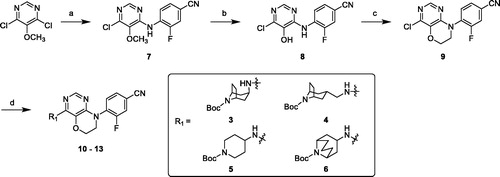
Scheme 1. Synthesis of compounds 10–13. Reagents and conditions: (a) 4-amino-3-fluorobenzonitrile, K2CO3, DMF, 65 °C, overnight. (b) 1 M BBr3 in DCM, anhydrous DCM, r. t. – reflux, 2 h. (c) 1-bromo-2-chloroethane, K2CO3, DMF, 40 °C, overnight. (d) amines 3–6, Pd2(dba)3, X-Phos, Cs2CO3, 1,4-Dioxane, reflux, under N2 overnight.
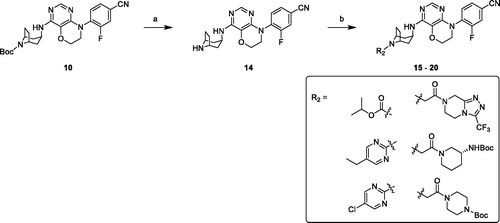
Scheme 2. Synthesis of compounds 15–20. Reagents and conditions: (a) 3 M HCl in EtOH, r. t., overnight. (b) chloro-fragments, Et3N, DCM, r. t., overnight or Cs2CO3, DMF, r. t., overnight.
Scheme 3. Synthesis of compounds 22–25. Reagents and conditions: (a) 3 M HCl in EtOH, r. t., overnight. (b) chloro-fragments, Et3N, DCM, r. t., overnight or Cs2CO3, DMF, r. t., overnight.
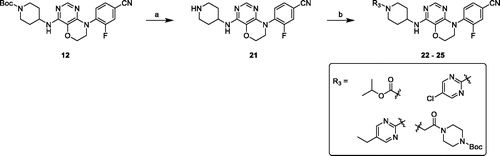
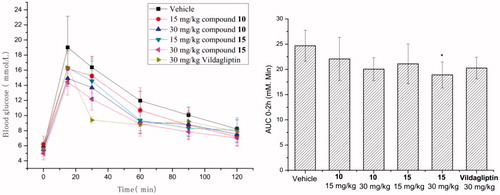
Table 1. GPR119 agonistic activities of compounds 10–13.
Table 2. GPR119 agonistic activities of compounds 15–20 and 22–25.