Figures & data
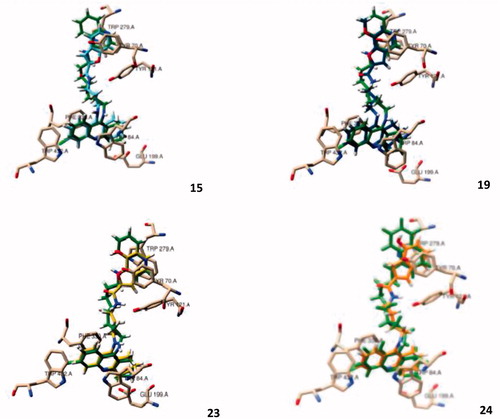
Figure 2. Molecular modelling of four representative compounds inside the binding site of AChE, superimposed with the original ligand (N-4′-quinolyl-N′-9″-(1″,2″,3″,4″-tetrahydroacridinyl)-1,8-diaminooctane), green Coloured. These compounds have the same TAC substituent (R = Cl), but different BF substituent (R1) and spacer length: 15 (turquoise Coloured; n = 1; R1 = H), 19 (dark blue Coloured; n = 1; R1 = -OCH3), 23 (yellow Coloured; n = 1; R1= -OH), 24 (orange Coloured; n = 2; R1 = H).
Scheme 1. Synthesis of TAC-BF hybrids. Reagents and conditions: (a) cyclohexanone, POCl3, 180 °C, 3 h; (b) DMF, K2CO3 (1.2 eq), ethylbromoacetate (1.05 eq), 135-140 °C, 5–6 h; (c) diaminoalkane (3.0 eq), dry MeOH, overnight; (d) phenol, KI, 165–170 °C 35–60 min; (e) BCl3, TBAI, DCM, under N2 (−78 °C), 2 h.
Figure 3. Species distribution curves for compound 21 along with molar extinction coefficients at the maximum absorption wavelengths (CL = 4.0 × 1 0 −5 M).
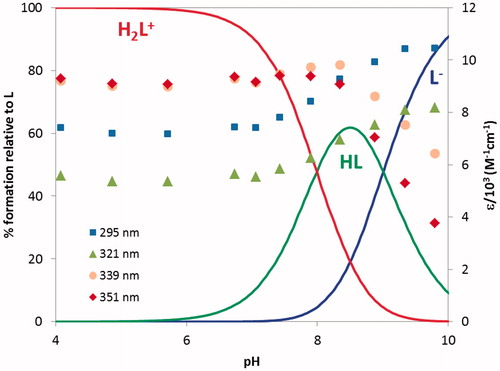
Table 1. Stepwise protonation constants for the ligand 21 and global formation constantsTable Footnotea for the corresponding Fe(III) and Cu(II)) complexes (T = 25.0 ± 0.1 °C, I = 0.1 M KCl, 25% w/w DMSO/water) as well as pMb values.
Figure 4. Species distribution curves for the systems (a) Fe(III)/21 1:3 and (b) Cu(II)/21 1:2 (CL = 4.0 × 1 0 −5 M).
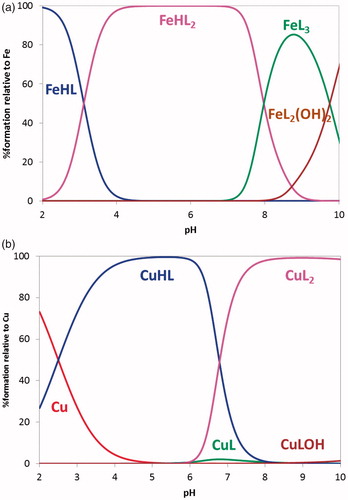
Figure 5. Graphical summary of the effect of different structural parameters on the inhibition of AChE (AChEi, IC50) and on the inhibition of Aβ aggregation (%): (A) size of alkylchain linker in AChEi: n = 1 (propylic chain, n = 2 (butylic chain); (B) substituents at C6 of TAC (R = H, Cl) in AChEi; (C) substituents at C7 of BF (R1 = H, -OCH3, -OH) in AChEi; (D) inhibition of Aβ aggregation (self- and Cu-induced).
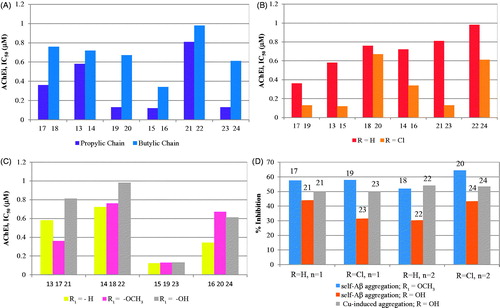
Table 2. Summary of results for the biological assays of TAC-BF hybrids
Figure 6. TEM images of Aβ aggregation inhibition performed with samples incubated (37 °C) for 24 h. Experimental conditions: [Aβ1–42] = [CuCl2] = 25 μM; [21] = 50 μM; pH = 6.6.
![Figure 6. TEM images of Aβ aggregation inhibition performed with samples incubated (37 °C) for 24 h. Experimental conditions: [Aβ1–42] = [CuCl2] = 25 μM; [21] = 50 μM; pH = 6.6.](/cms/asset/3401332d-0520-42f1-a56b-8c11f78284ce/ienz_a_1689237_f0006_b.jpg)
Table 3. Summary of calculated pharmacokinetic molecular descriptors for the new hybrids by QikProp v.2.54039
Figure 7. Dose-response screening to select non-toxic concentrations of TAC-BF hybrids. Cells were treated with varying concentration of TAC-BF conjugates (from 10 μM to 40 μM) for 25 h and cell viability was determined using the MTT reduction assay. Results are expressed relatively to SH-SY5Y untreated cells, with the mean ± SEM derived from three different experiments. *p < 0.05; **p < 0.01, significantly different when compared with SH-SY5Y untreated cells.
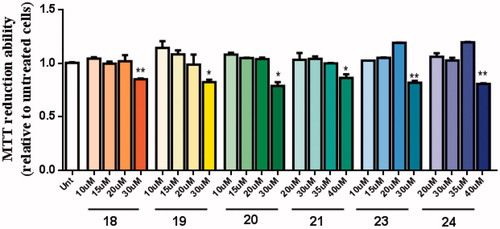
Figure 8. Neuroprotective effect of TAC-BF hybrids from Aβ42-induced toxicity on SH-SY5Y cells. Cells were treated with Aβ42 peptide (2.5 μM) for 24 h in the absence or in the presence of the compounds (1 h pre-incubation + 24 h co-incubation). Evaluation of cell viability was performed using the MTT reduction assay. Results are expressed relatively to SH-SY5Y untreated cells, with the mean ± SEM derived from 4 different experiments. *p < 0.05, significantly different when compared with SH-SY5Y untreated cells; #p < 0.05, significantly different when compared with Aβ42 treated SH-SY5Y cells. (compounds: 18, 19, 20 and 23 – 20 µM; 21, 24 – 35 µM).
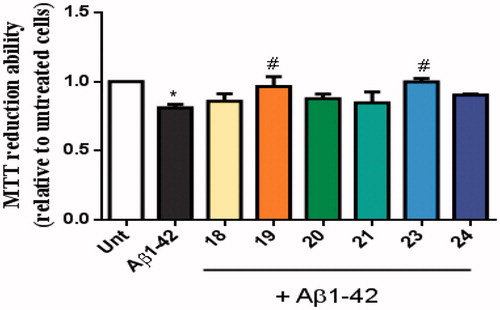
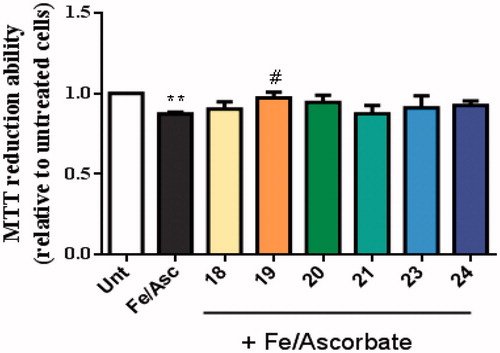
Figure 9. Neuroprotective effect of TAC-BF conjugates against L-Ascorbic Acid (AscH(-))/Ferrous Sulphate (Fe) toxicity on SH-SY5Y cells. Cells were treated with Asc/Fe (5 mM/500 μM, respectively) for 24 h, after treatment for 1 h in the absence or in the presence of the compounds. Evaluation of cell viability was performed by using MTT reduction assay. Results are expressed relatively to SH-SY5Y untreated cells, with the mean ± SEM derived from 4 different experiments. **p < 0.01, significantly different when compared with SH-SY5Y untreated cells. #p < 0.05, significantly different when compared with Asc/Fe treated SH-SY5Y cells. (compounds: 18, 19, 20 and 23 – 20 µM; 21, 24 – 35 µM).