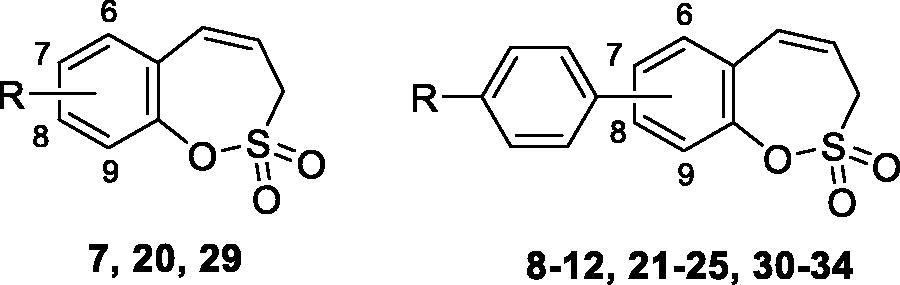
Figures & data
Scheme 1. Reagents and conditions for the preparation of derivatives 8–12: (i) ICl, AcOH, 40 °C, 24 h, 84%; (ii) KOtBu, CH3P(C6H5)3Br, THF, RT, 18 h, 83%; (iii) NEt3, CH2Cl2, 0 °C to RT, 4 h, 83%; (iv) toluene, 70 °C, 4 h, 89%; (v) Ar-B(OH)2, Pd(PPh3)4, K3PO4, toluene/H2O, 100 °C, 16 h.
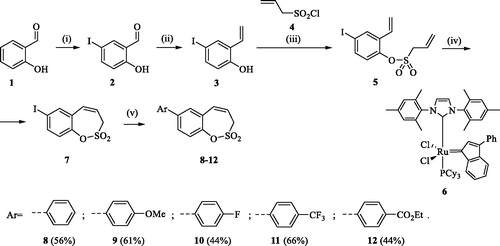
Scheme 2. Reagents and conditions: (i) KOtBu, CH3P(C6H5)3Br, THF, RT, 18 h, 82%; (ii) 4, NEt3, CH2Cl2, 0 °C to RT, 4 h, 66%; (iii) a) 6 (5 mol% and 10 mol%), toluene, 70 °C, 40 h, 0%; b) Schrock catalyst [Mo] (10 mol%), toluene, 70 °C, 16 h, 0%; c) Schrock–Hoveyda [Mo] (10 mol%), toluene, 70 °C, 16 h, 0%;
![Scheme 2. Reagents and conditions: (i) KOtBu, CH3P(C6H5)3Br, THF, RT, 18 h, 82%; (ii) 4, NEt3, CH2Cl2, 0 °C to RT, 4 h, 66%; (iii) a) 6 (5 mol% and 10 mol%), toluene, 70 °C, 40 h, 0%; b) Schrock catalyst [Mo] (10 mol%), toluene, 70 °C, 16 h, 0%; c) Schrock–Hoveyda [Mo] (10 mol%), toluene, 70 °C, 16 h, 0%;](/cms/asset/7085906e-c1a3-4615-8d8d-f2d717978f86/ienz_a_1695795_sch0002_b.jpg)
Scheme 3. Reagents and conditions: (i) KOtBu, CH3P(C6H5)3Br, THF, RT, 18 h, 76%; (ii) 4, NEt3, CH2Cl2, 0 °C to RT, 4 h, 54%; (iii) 6, toluene, 70 °C, 4 h, 90%; (iv) Ar-B(OH)2, Pd(PPh3)4, K3PO4, toluene/H2O, 100 °C, 16 h.
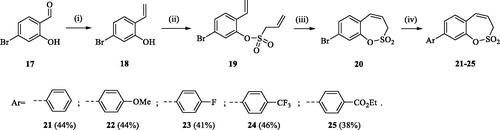
Scheme 4. Reagents and conditions: (i) KOtBu, CH3P(C6H5)3Br, THF, RT, 18 h, 80%; (ii) 4, NEt3, CH2Cl2, 0 °C to RT, 4 h, 86%; (iii) 6, toluene, 70 °C, 4 h, 78%; (iv) Ar-B(OH)2, Pd(PPh3)4, K3PO4, toluene/H2O, 100 °C, 16 h.
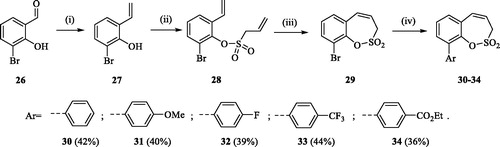
Table 1. Inhibition data of human CA isoforms CA I, II, IX and XII with 3H-1,2-benzoxathiepines 2,2-dioxide 7–34 using AAZ as a standard drug.