Figures & data
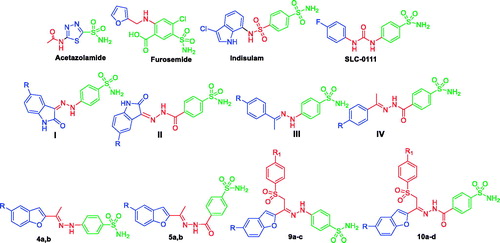
Figure 1. Structures of some CAIs, and the target benzofuran-based sulphonamides 4a, b, 5a, b, 9b–d and 10a–d.
Scheme 2. Reagent and conditions: (i) Br2/Acetic Acid, Stirring at r.t 4 h; (ii) Abs.Ethanol, reflux 4 h; (iii) Ethanol/Acetic acid, reflux 4 h.
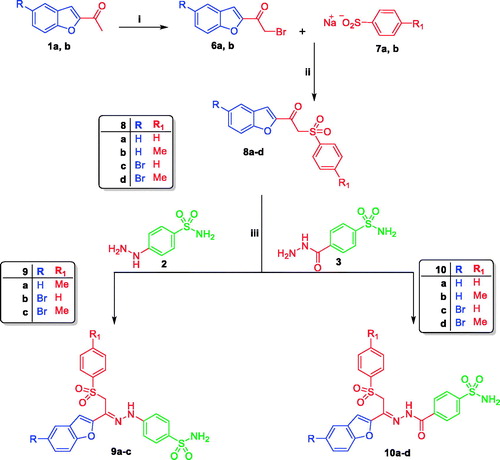
Table 1. Inhibition data of human CA isoforms hCA I, II, IX and XII for the target sulphonamides (4a,b, 5a,b, 9a–c, and 10a–d), using (AAZ) as a standard drug.
Table 2. Selectivity ratios for the inhibition of hCA IX and XII over hCA I and II for targeted compounds 4a, b, 5a, b, 9a–c and 10a–d.