Figures & data
Table 1. Inhibitory activity (MIC, μg/mL) of compounds 3a–3j and 6a–6k against Gram-positive bacteria and Gram-negative bacteria.
Table 2. Inhibitory activity (MIC, µg/mL) of compounds 3c–3e, 3g, 3i, 3j, 6a, 6b and 6e against clinical isolates of multidrug-resistant strains.
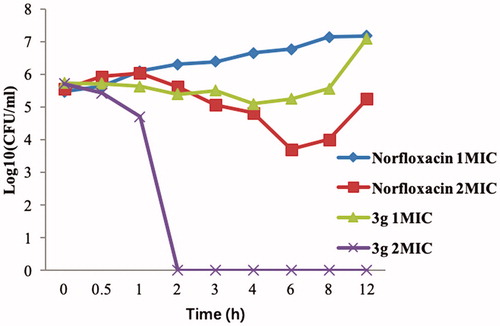
Figure 2. Propensity of the development of bacterial resistance towards compound 3g by (A) S. aureus and (B) E. coli.
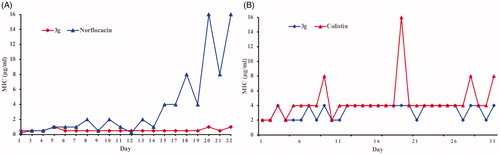
Table 3. The Inhibitory activity (IC50, µg/mL) of compounds 3e, 3f, 3g, 3i, 6a, 6b, 6e and 6k against cancer cell lines A549 and SGC7901 and normal cell lines L02.