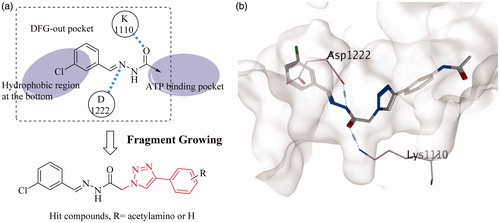
Figures & data
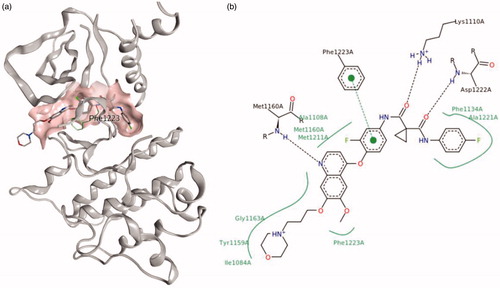
Figure 1. Structure of the kinase domain of c-Met bind to GSK1363089, (a) 3D-view image; (b) pose-view image.
Scheme 1. Reagents and conditions: (i) CuSO4, Vitamin C, EtOH/H2O, 40 °C, 5–10 h; (ii) NaOH, 6 h, HCl; (iii) hydrazine hydrate, rt, 5 h; (iv) EDCI, HOBt, Et3N, 35 °C, 8–10 h.
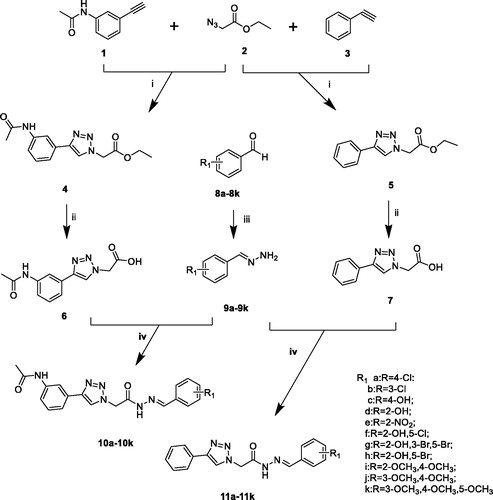
Table 1. Novel compounds and their activities against c-Met.
Table 2. Descriptors and relative importance of descriptors.
Figure 3. Important structural requirements of (E)-N'-benzylidene hydrazides by means of the ligand based 3D-QSAR.
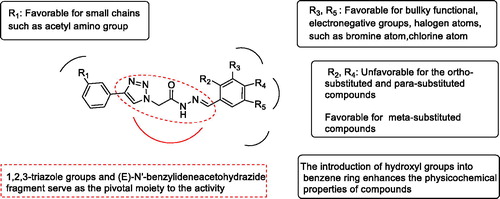
Table 3. The inhibitory activities of the five compounds against VEGFR-2 and c-Met.