Figures & data
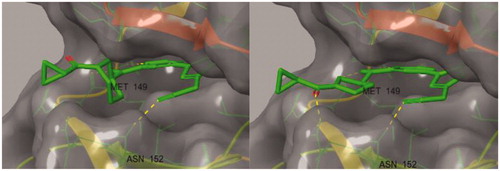
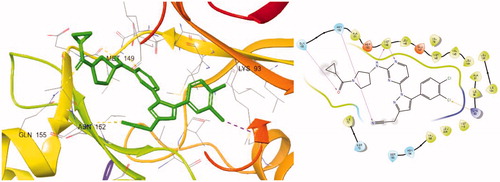
Figure 1. Docking structures of the previous JNK3 inhibitor (PDB: 3OY1) and design of the present 1-pyrimidyl-3-alkyl-5-aryl-1H-pyrazole scaffold.
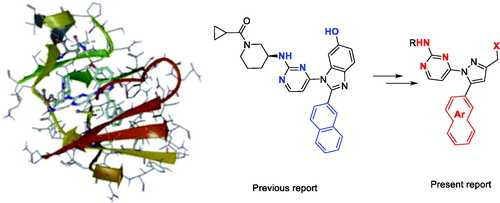
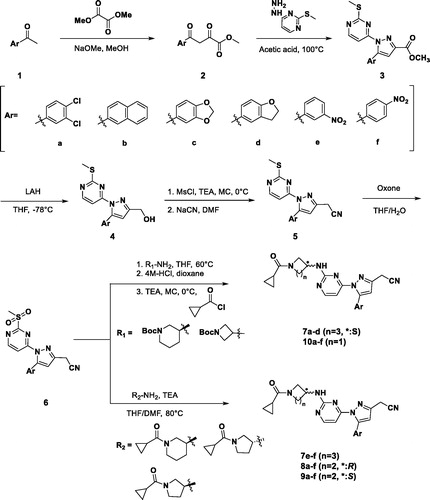
Table 1. Enzymatic activities of 1-heteroaryl-3-alkyl-5-aryl-1H-pyrazole derivatives. 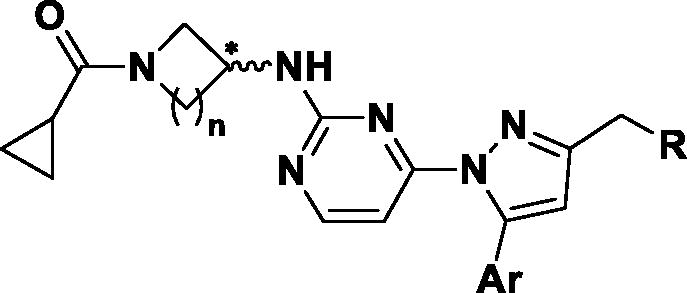
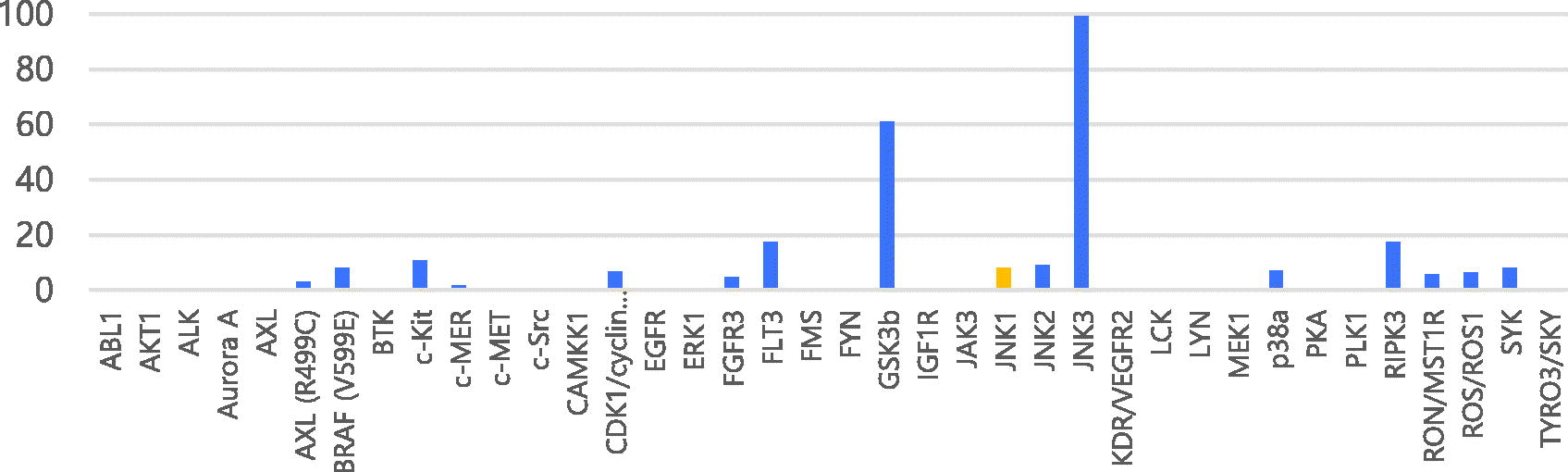
Table 2. Percentages of enzymatic inhibition exerted by 7a (10 μM) on 38 selected protein kinases and enzymatic activities on selected protein kinases.