Figures & data
Scheme 2. Photochemical reactivity of amino-5-arylethenyl-oxazoles trans-2,6,18 into naphtho[1,2-d]oxazoles, 19,20 and 21, respectively.
![Scheme 2. Photochemical reactivity of amino-5-arylethenyl-oxazoles trans-2,6,18 into naphtho[1,2-d]oxazoles, 19,20 and 21, respectively.](/cms/asset/a3d58b7f-f1f9-4fab-8e86-903a50253e70/ienz_a_1707197_sch0002_c.jpg)
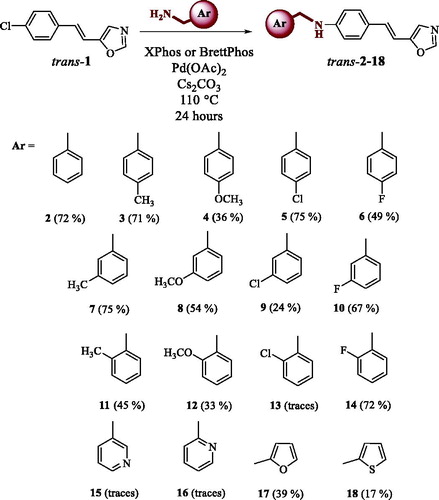
Figure 1. UV spectra of compounds trans-2 and trans-17 (a), para-substituted synthesised compounds trans-3–6 (b), meta-substituted synthesised compounds trans-7–10 (c) and ortho-substituted synthesised compounds trans-11, trans-12 and trans-14 (d).
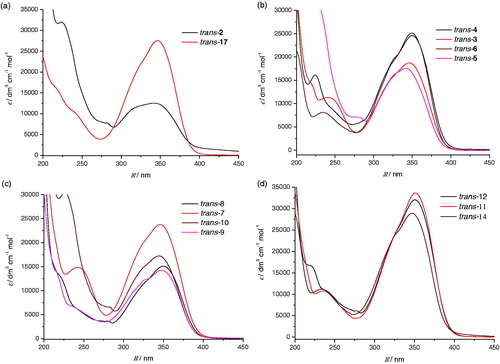
Figure 2. Partial 1H NMR spectra of starting amines trans-2 and trans-17 and of the photocyclization product 19.
Table 1. Inhibition of BChE and AChE by tested trans-amino-5-arylethenyl-oxazole derivatives (trans-2-trans-17), naphtho[1,2-d]oxazoles (19–21 and 23), and amino-4/5-arylethenyl-oxazoles (cis-18 and cis-22), expressed as IC50 ± SE.