Figures & data
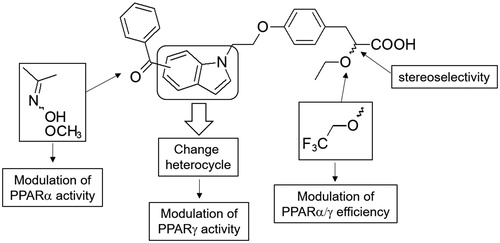
Figure 1. Design of α–alkoxyphenylpropionic acid derivatives bearing various central nitrogen heterocycles.
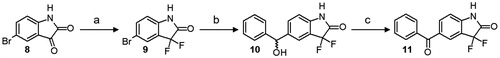
Scheme 1. Synthesis of 5-benzoyl-3,3-difluoro-1,3-dihydro-2H-indol-2-one 11. Reagents and conditions: (a) DAST, DCM, r.t.; (b) i: i-PrMgCl, n-BuLi, dry THF, 0 °C; ii: benzaldehyde, −78 °C; (c) Dess-Martin periodinane, DCM, r.t.
Scheme 2. Synthesis of optically pure α–alkoxyphenylpropionic acids 21, 22. Reagents and conditions: (a) ethanol or trifluoroethanol [Rh(OAc)2]2,toluene, reflux; (b) 1,2-bromo-chloroethane, K2CO3, CH3CN, reflux; (c) NaH, dry THF, 0 °C to r.t.; (d) H2, Pd/C, ethanol, r.t.; (e) LiOH, THF/H2O, r.t.; (f) 1-ethyl-3–(3-dimethylaminopropyl)-carbodiimide hydrochloride, triethylamine, HOBT, (S)-(-)-2-phenylglycinol, DCM, 0 °C to r.t.; (g) 5 M H2SO4, dioxane/H2O, reflux.
![Scheme 2. Synthesis of optically pure α–alkoxyphenylpropionic acids 21, 22. Reagents and conditions: (a) ethanol or trifluoroethanol [Rh(OAc)2]2,toluene, reflux; (b) 1,2-bromo-chloroethane, K2CO3, CH3CN, reflux; (c) NaH, dry THF, 0 °C to r.t.; (d) H2, Pd/C, ethanol, r.t.; (e) LiOH, THF/H2O, r.t.; (f) 1-ethyl-3–(3-dimethylaminopropyl)-carbodiimide hydrochloride, triethylamine, HOBT, (S)-(-)-2-phenylglycinol, DCM, 0 °C to r.t.; (g) 5 M H2SO4, dioxane/H2O, reflux.](/cms/asset/6d360411-607e-4cb1-b8d3-9f338c76a671/ienz_a_1713771_sch0002_b.jpg)
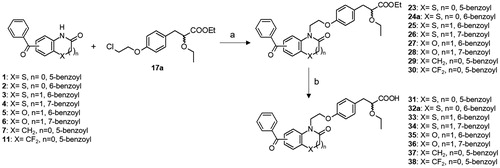
Scheme 3. Synthesis of PPAR agonists 31–38. Reagents and conditions: (a) K2CO3, DMF, 120 °C; (b) LiOH, THF/H2O, r.t.
Scheme 4. Synthesis of optically pure and oxime derived PPAR agonists 39–46. Reagents and conditions: (a) K2CO3, 17a or 17 b, DMF, 120 °C; (b) LiOH, THF/H2O, r.t; (c) hydroxylamine hydrochloride or O-methylhydroxylamine hydrochloride, pyridine, reflux; (d) NaH, 21a,b or 22a,b, HMPA, r.t.
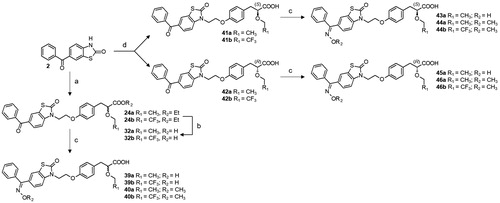
Table 1. In vitro profile of compounds 31–38 on PPARγ affinity and PPARα/γ transactivation.
Table 2. In vitro profile of compounds 32, 39–46 on PPARγ affinity and PPARα/γ transactivation.
Figure 2. Predicted binding mode of (A) (S)-34 (pink) and (S)-36 (cyan) and (C) (S)-33 (pink) and (S)-35 (cyan) enantiomers with PPARγ LBD (PDB id 1I7I). Key amino acid side chains are shown in white stick format and are labelled and hydrogen bonds are shown in red dotted lines. Overlapping and molecular surface maps of (B) (S)-34 (pink) and (S)-36 (cyan) and (D) (S)-33 (pink) and (S)-35 (cyan) enantiomers with PPARγ LBD (PDB id 1I7I). The protein and key amino acid side chains are shown in white rounded ribbon and white stick format, respectively. The molecular surface maps are shown in grey.
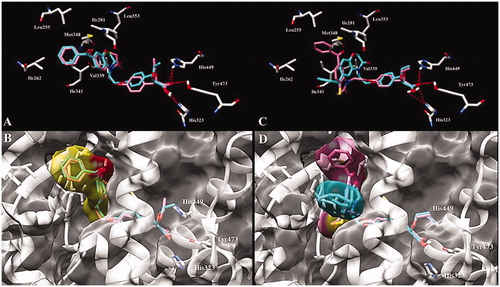
Table 3. In vivo efficacy of compounds 39a,b, 40a,b, 43a, 44a,b, 45a in ob/ob mice animal models.
