Figures & data
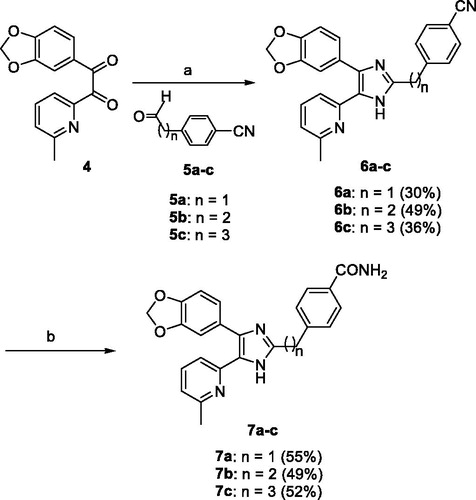
Scheme 1. Reagents and conditions: (a) NH4OAc, AcOH, 120 °C, 3 h; (b) 28% H2O2, 6 N NaOH, EtOH, 55 °C, 3 h.
Scheme 2. Reagents and conditions: (a) NaNO2, 5 N HCl, rt, 1 h; (b) 5a or 3-(2-oxoethyl)benzonitrile (5d), NH4OAc, t-BuOMe/MeOH, rt, overnight, Ar atmosphere; (c) (i) 28% H2O2, 6 N NaOH, EtOH, DMSO, 55 °C, overnight; (ii) triethyl phosphite, anhydrous DMF, 110 °C, 72 h.
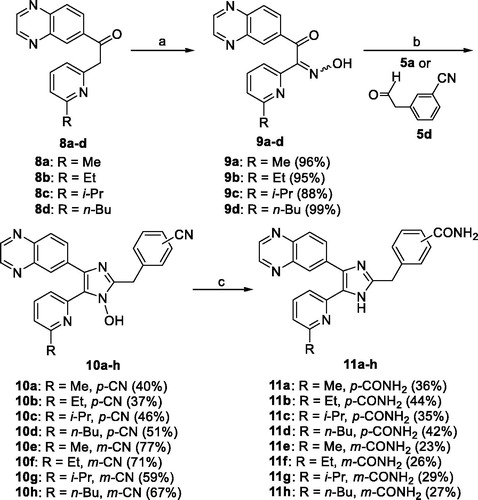
Scheme 3. Reagents and conditions: (a) 28% H2O2, 6 N NaOH, MeOH, 55 °C, 2 h; (b) 1 N HCl, THF, reflux, 1 h.
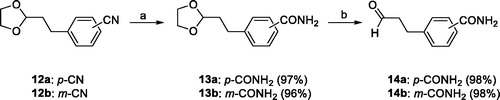
Scheme 4. Reagents and conditions: (a) 14a or 14 b, NH4OAc, t-BuOMe/MeOH, 30 °C, overnight, Ar atmosphere.
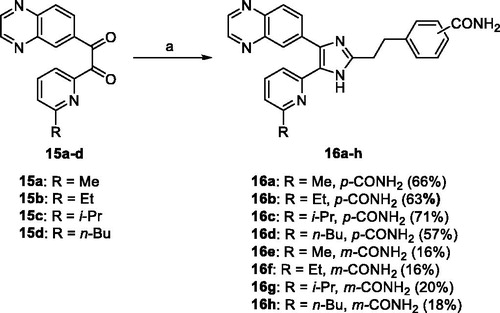
Table 1. ALK5 inhibitory activity and Caco-2 cell permeability of 2,4-disubstitubed-5–(6-alkylpyridin-2-yl)-1H-imidazoles 7a–c, 11a–h, and 16a–h.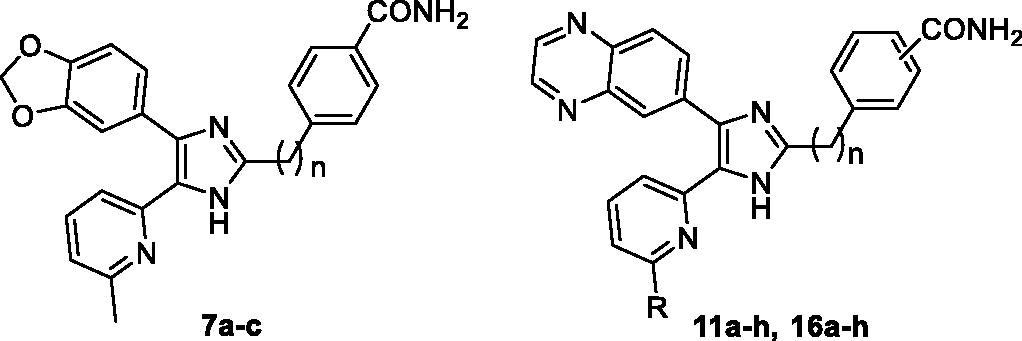
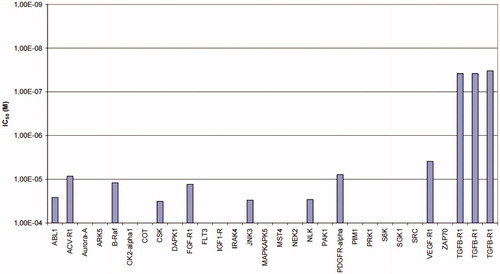
Table 2. ALK5 and p38α inhibitory activity of 11e, 1, and 2.
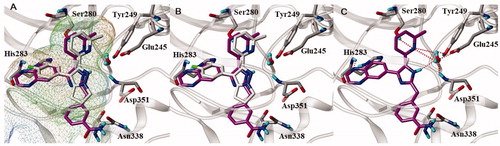
Figure 3. Docked pose of 11e in the active site of ALK5 (PDBid:1RW8). (A, B) 11e is (magenta carbon atoms) superimposed over the X-ray pose of native ligand (grey carbon atoms). The active site of ALK5 is shown as MOLCAD lipophilic potential surface map (A), and lipophilicity increases from blue (hydrophilic) to brown (liphophilic). Grey capped sticks represent key amino acid residues within the binding site, and the backbone of ALK5 is shown as ribbon. The bound water molecule in the X-ray structure is represented by ball and stick. (C) Intermolecular interaction between 11e and ALK5. Grey capped sticks represent key amino acid residues in the active site. Red dashed lines are hydrogen bonding interactions (<3.0 Å).