Figures & data
Scheme 1. General synthetic route to 3-arylbenzofuranone, reagents and conditions: (a) CHCl3, TBAB, NaOH, 40–50 °C; (b) H3O+; (c) BF3·Et2O, 30 °C.
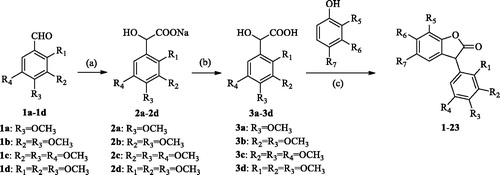
Table 1. Compounds 1–23.
Table 2. Biological evaluation in vitro.
Figure 1. Kinetic study of the mechanism of ChEs inhibition by compound 13. Overlaid Lineweaver–Burk reciprocal plots of ChEs initial velocity at increasing substrate concentration in the absence of inhibitor and in the presence of 13 are shown. A is a double reciprocal plot of compound 13 inhibition of AChE. B is a double reciprocal plot of compound 13 inhibition of BuChE.
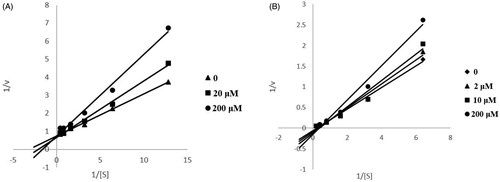
Figure 2. Schematic presentations of the putative AChE binding modes with compound 20 and compound 11.
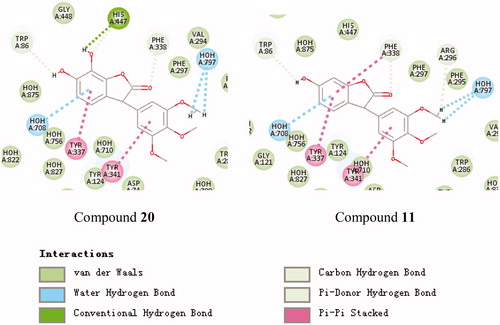
Figure 3. Schematic presentations of the putative BuChE binding modes with compound 13 and compound 19.
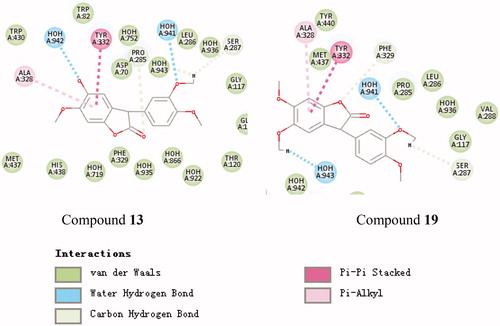
Figure 4. Schematic presentations of the putative MAO-B binding modes with compound 9 and compound 12.
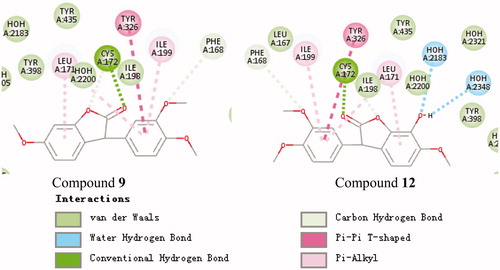
Table 3. TOPKAT prediction results
