Figures & data
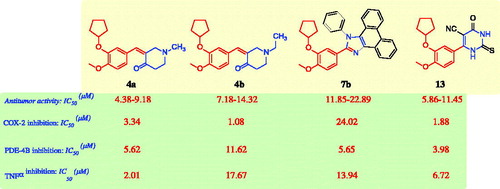
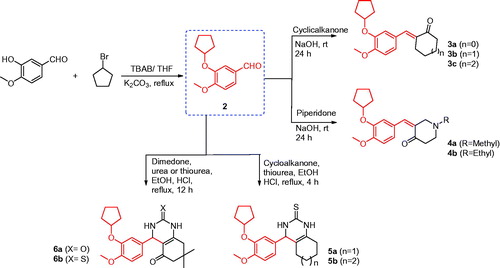
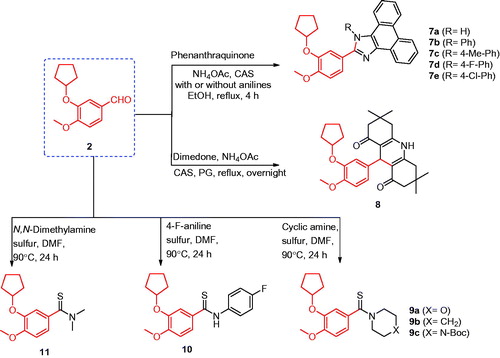
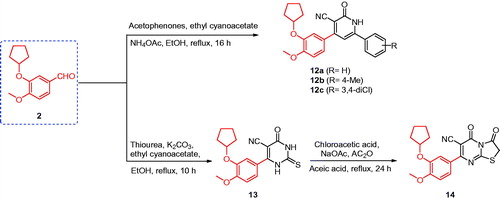
Figure 1. The structures of the reported antitumor agents (A–F) with COX-2 or PDE4 and the designed compounds 3–14.
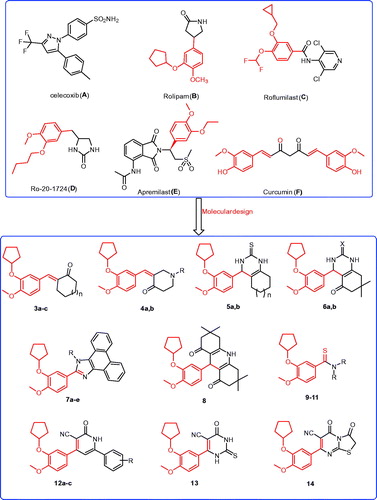
Table 1. In vitro antitumor activity of the designed compounds, celecoxib, afatinib, and doxorubicin against human tumour cells.
Table 2. In vitro inhibitory effects of COX-2, PDE-4B, and TNF-α of the antitumor compounds 4a, 4b, 7b, and 13.Table Footnotea
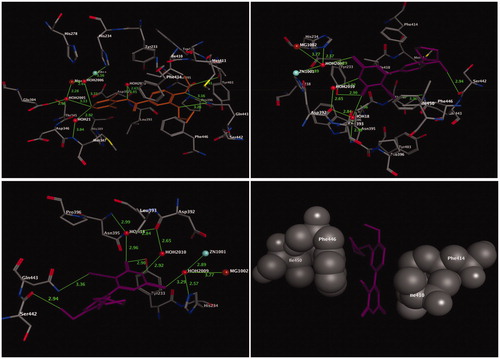
Figure 2. Three-dimensional (3D) orientation of the docked ligand SC-558 (upper left panel); docked compounds 4b (lower left panel), and 13 (lower right panel) in the active pocket of the COX-2 enzyme (H bond interactions are shown as green lines). Upper right panel showed the alignment of SC-558, 4b, and 13 in the active pocket of the COX-2 enzyme.
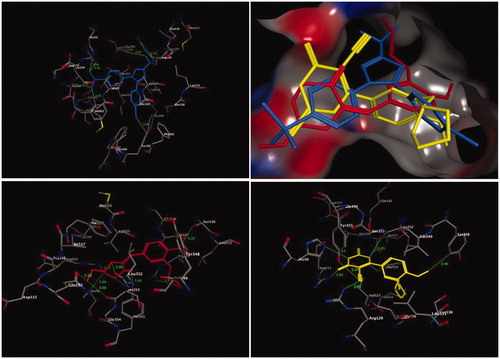
Figure 3. Three-dimensional (3D) orientation of the docked roflumilast (upper left panel); docked compound 13 (upper right panel), in the active pocket of the PDE4B enzyme (H bond interactions are shown as green lines). Lower left panel showed near picture of compound 13 in the active pocket of the PDE4B enzyme. Lower right panel showed the hydrophobic interactions of compound 13 in the active pocket of the PDE4B enzyme.