Figures & data
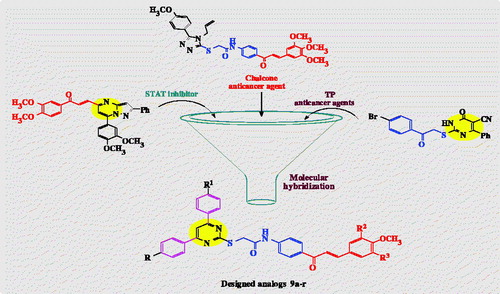
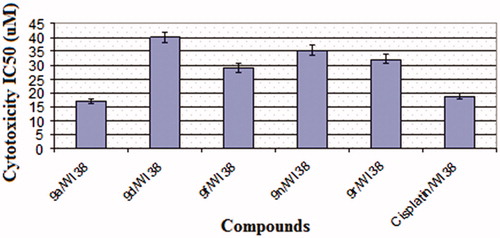
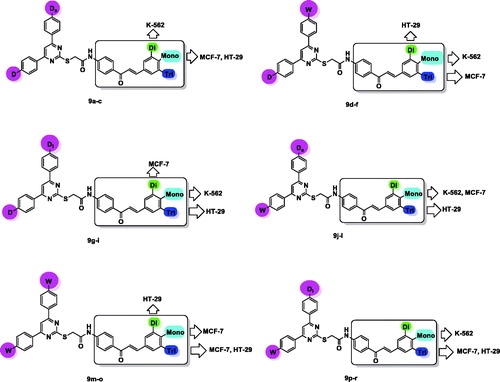
Table 1. Cytotoxicity results of pyrimidine/chalcone hybrids 9a–r against three different cancer cell lines.
Table 2. STAT3 and STAT5a inhibitory activity of compounds 9a, 9d, 9f, 9n, 9r and reference drug pacritinib.
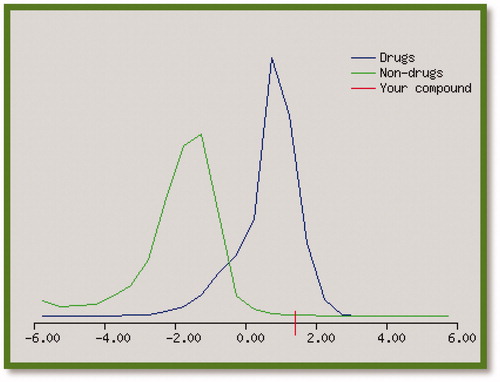
Table 3. Biological properties, prediction, and drug likeness of the target compounds.
Table 4. Pharmacokinetic properties assessment of the target synthesised compounds 9a–r.
Table 5. Toxicity assessment of the target synthesised compounds 9a–r.